The Receptor for Advanced Glycation Endproducts (RAGE) Contributes to Severe Inflammatory Liver Injury in Mice
- PMID: 32670276
- PMCID: PMC7326105
- DOI: 10.3389/fimmu.2020.01157
The Receptor for Advanced Glycation Endproducts (RAGE) Contributes to Severe Inflammatory Liver Injury in Mice
Abstract
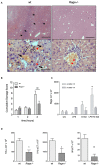
Background: The receptor for advanced glycation end products (RAGE) is a multiligand receptor involved in a number of processes and disorders. While it is known that RAGE-signaling can contribute to toxic liver damage and fibrosis, its role in acute inflammatory liver injury and septic multiorgan failure is yet undefined. We examined RAGE in lipopolysaccharide (LPS) induced acute liver injury of D-galN sensitized mice as a classical model for tumor necrosis factor alpha (TNF-α) dependent inflammatory organ damage. Methods: Mice (Rage-/- and C57BL/6) were intraperitoneally injected with D-galN (300 mg/kg) and LPS (10 μg/kg). Animals were monitored clinically, and cytokines, damage associated molecular pattern molecules (DAMPs) as well as liver enzymes were determined in serum. Liver histology, hepatic cytokines as well as RAGE mRNA expression were analyzed. Cellular activation and functionality were evaluated by flow cytometry both in bone marrow- and liver-derived cells. Results: Genetic deficiency of RAGE significantly reduced the mortality of mice exposed to LPS/D-galN. Hepatocyte damage markers were reduced in Rage-/- mice, and liver histopathology was less severe. Rage-/- mice produced less pro-inflammatory cytokines and DAMPs in serum and liver. While immune cell functions appeared normal, TNF-α production by hepatocytes was reduced in Rage-/- mice. Conclusions: We found that RAGE deletion attenuated the expression of pro-inflammatory cytokines and DAMPs in hepatocytes without affecting cellular immune functions in the LPS/D-galN model of murine liver injury. Our data highlight the importance of tissue-specific RAGE-signaling also in acute inflammatory liver stress contributing to sepsis and multiorgan failure.
Keywords: DAMPs; PAMPs; RAGE; inflammatory liver injury; sepsis.
Copyright © 2020 Weinhage, Wirth, Schütz, Becker, Lueken, Skryabin, Wittkowski and Foell.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

