Development of a Direct in vitro Plant Regeneration Protocol From Cannabis sativa L. Seedling Explants: Developmental Morphology of Shoot Regeneration and Ploidy Level of Regenerated Plants
- PMID: 32670304
- PMCID: PMC7326123
- DOI: 10.3389/fpls.2020.00645
Development of a Direct in vitro Plant Regeneration Protocol From Cannabis sativa L. Seedling Explants: Developmental Morphology of Shoot Regeneration and Ploidy Level of Regenerated Plants
Abstract
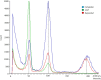
In vitro shoot regeneration can efficiently contribute to the improvement of recalcitrant Cannabis sativa L. We aimed at developing a highly efficient protocol for in vitro direct regeneration of C. sativa plants from different explants (cotyledon, hypocotyl, and true leaf) from seedlings of monoecious C. sativa short-day varieties Ferimon, Felina32, Fedora17, and USO31, together with dioecious neutral-day variety Finola. Ten regeneration media, including already published protocols, and self-designed combinations of plant growth regulators were tested. The developmental morphology since germination of seeds to the development of rooted plantlets was followed. Additionally, the ploidy level of explants and in vitro regenerants was analyzed. We concluded that hypocotyl is the best explant for in vitro direct regeneration of C. sativa plants with 49.45% of responding explants, while cotyledon and true leaf had a poor response with, respectively, 4.70 and 0.42% of explants developing plantlets. In terms of shoot regeneration, we found significant differences among the culture media evaluated and the varieties studied. Overall, the best regeneration media were ZEARIB 2.0 (mg/L) and ZEARIB 1.0 (mg/L) + NAA 0.02 (mg/L) with 66.67% of responding hypocotyls. Amazingly, hypocotyls cultured in medium without plant growth regulators showed an excellent response (61.54% of responding hypocotyls) and spontaneous rooting of regenerants (17.94%). In vitro regenerated plants were acclimatized just 6 weeks after culture initiation. The developmental morphology study suggests that regenerated shoots originate from pericycle cells adjacent to xylem poles. Polysomaty was detected in hypocotyls and cotyledons of all varieties studied, and diploid (>80%) and mixoploid (with diploid and tetraploid cells) plants were regenerated. Our protocol allows a high shoot organogenesis efficiency in different C. sativa varieties. The fact that a significant percentage of plants are mixoploid may provide an alternative way to develop polyploids in C. sativa. Our results show that direct in vitro regeneration may make a significant contribution to the development of improved C. sativa materials for medical applications.
Keywords: cannabinoids; hemp; hypocotyl; micropropagation; polyploidization; polysomaty; shoot organogenesis.
Copyright © 2020 Galán-Ávila, García-Fortea, Prohens and Herraiz.
Figures







References
-
- Adelberg J. W., Rhodes, Bill B., Skorupska H. T. (1993). Generating tetraploid melons in tissue culture. Acta Hortic. 336 373–380. 10.17660/actahortic.1993.336.49 - DOI
-
- Beck E. H. (1996). Regulation of shoot/root ratio by cytokinins from roots in Urtica dioica: opinion. Plant Soil 185 3–12. 10.1007/bf02257560 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

