ATM Promotes RAD51-Mediated Meiotic DSB Repair by Inter-Sister-Chromatid Recombination in Arabidopsis
- PMID: 32670319
- PMCID: PMC7329986
- DOI: 10.3389/fpls.2020.00839
ATM Promotes RAD51-Mediated Meiotic DSB Repair by Inter-Sister-Chromatid Recombination in Arabidopsis
Abstract
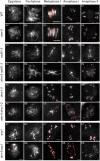
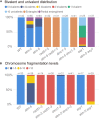
Meiotic recombination ensures accurate homologous chromosome segregation during meiosis and generates novel allelic combinations among gametes. During meiosis, DNA double strand breaks (DSBs) are generated to facilitate recombination. To maintain genome integrity, meiotic DSBs must be repaired using appropriate DNA templates. Although the DNA damage response protein kinase Ataxia-telangiectasia mutated (ATM) has been shown to be involved in meiotic recombination in Arabidopsis, its mechanistic role is still unclear. In this study, we performed cytological analysis in Arabidopsis atm mutant, we show that there are fewer γH2AX foci, but more RAD51 and DMC1 foci on atm meiotic chromosomes. Furthermore, we observed an increase in meiotic Type I crossovers (COs) in atm. Our genetic analysis shows that the meiotic phenotype of atm rad51 double mutants is similar to the rad51 single mutant. Whereas, the atm dmc1 double mutant has a more severe chromosome fragmentation phenotype compared to both single mutants, suggesting that ATM functions in concert with RAD51, but in parallel to DMC1. Lastly, we show that atm asy1 double mutants also have more severe meiotic recombination defects. These data lead us to propose a model wherein ATM promotes RAD51-mediated meiotic DSB repair by inter-sister-chromatid (IS) recombination in Arabidopsis.
Keywords: ATM; DSBs; RAD51; inter-sister chromatids; meiosis; recombination.
Copyright © 2020 Yao, Li, Chen, Liu, Mi, Ren, Mo and Lu.
Figures






References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

