Insecticidal Activity of Artemisia vulgaris Essential Oil and Transcriptome Analysis of Tribolium castaneum in Response to Oil Exposure
- PMID: 32670352
- PMCID: PMC7330086
- DOI: 10.3389/fgene.2020.00589
Insecticidal Activity of Artemisia vulgaris Essential Oil and Transcriptome Analysis of Tribolium castaneum in Response to Oil Exposure
Abstract
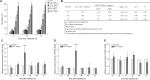
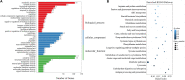
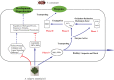
Red flour beetle (Tribolium castaneum) is one of the most destructive pests of stored cereals worldwide. The essential oil (EO) of Artemisia vulgaris (mugwort) is known to be a strong toxicant that inhibits the growth, development, and reproduction of T. castaneum. However, the molecular mechanisms underlying the toxic effects of A. vulgaris EO on T. castaneum remain unclear. Here, two detoxifying enzymes, carboxylesterase (CarEs) and cytochrome oxidase P450 (CYPs), were dramatically increased in red flour beetle larvae when they were exposed to A. vulgaris EO. Further, 758 genes were differentially expressed between EO treated and control samples. Based on Gene Ontology (GO) analysis, numerous differentially expressed genes (DEGs) were enriched for terms related to the regulation of biological processes, response to stimulus, and antigen processing and presentation. Our results indicated that A. vulgaris EO disturbed the antioxidant activity in larvae and partially inhibited serine protease (SP), cathepsin (CAT), and lipase signaling pathways, thus disrupting larval development and reproduction as well as down-regulating the stress response. Moreover, these DEGs showed that A. vulgaris indirectly affected the development and reproduction of beetles by inducing the expression of genes encoding copper-zinc-superoxide dismutase (CuZnSOD), heme peroxidase (HPX), antioxidant enzymes, and transcription factors. Moreover, the majority of DEGs were mapped to the drug metabolism pathway in the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. Notably, the following genes were detected: 6 odorant binding proteins (OBPs), 5 chemosensory proteins (CSPs), 14 CYPs, 3 esterases (ESTs), 5 glutathione S-transferases (GSTs), 6 UDP-glucuronosyltransferases (UGTs), and 2 multidrug resistance proteins (MRPs), of which 8 CYPs, 2 ESTs, 2 GSTs, and 3 UGTs were up-regulated dramatically after exposure to A. vulgaris EO. The residual DEGs were significantly down-regulated in EO exposed larvae, implying that partial compensation of metabolism detoxification existed in treated beetles. Furthermore, A. vulgaris EO induced overexpression of OBP/CYP, and RNAi against these genes significantly increased mortality of larvae exposed to EO, providing further evidence for the involvement of OBP/CYP in EO metabolic detoxification in T. castaneum. Our results provide an overview of the transcriptomic changes in T. castaneum in response to A. vulgaris EO.
Keywords: Artemisia vulgaris; Tribolium castaneum; development; insecticidal activity; metabolism system; reproduction.
Copyright © 2020 Gao, Zhang, Wei, Wei, Xiong, Lu, Zhang, Gao and Li.
Figures

References
-
- Alizadeh M., Aghaei M., Sharifian I., Saadatian M. (2012). Chemical composition of essential oil of Artemisia vulgaris from West Azerbaijan, Iran. Electron. J. Environ. Agric. Food Chem. 11 493–496.
-
- Aravindan V., Muthukumaravel S., Gunasekaran K. (2014). Interaction affinity of delta and epsilon class glutathione-s-transferases (GSTs) to bind with DDT for detoxification and conferring resistance in Anopheles gambiae, a malaria vector. J. Vector Borne Dis. 51 8–15. 10.1051/parasite/2014008 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

