Diels-Alder Reactivity of a Chiral Anthracene Template with Symmetrical and Unsymmetrical Dienophiles: A DFT Study
- PMID: 32670739
- PMCID: PMC7349856
- DOI: 10.1002/open.202000137
Diels-Alder Reactivity of a Chiral Anthracene Template with Symmetrical and Unsymmetrical Dienophiles: A DFT Study
Abstract
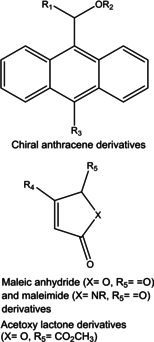
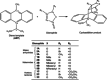
In this work, we used Density Functional Theory calculations to assess the factors that control the reactivity of a chiral anthracene template with three sets of dienophiles including maleic anhydrides, maleimides and acetoxy lactones in the context of Diels-Alder cycloadditions. The results obtained here (at the M06-2X/6-311++G(d,p) level of theory) suggest that the activation energies for maleic anhydrides and acetoxy lactones are dependent on the nature of the substituent in the dienophile. Among all studied substituents, only -CN reduces the energy barrier of the cycloaddition. For maleimides, the activation energies are independent of the heteroatom of the dienophile and the R group attached to it. The analysis of frontier molecular orbitals, charge transfer and the activation strain model (at the M06-2X/TZVP level based on M06-2X/6-311++G(d,p) geometries) suggest that the activation energies in maleic anhydrides are mainly controlled by the amount of charge transfer from the diene to the dienophile during cycloaddition. For maleimides, there is a dual control of interaction and strain energies on the activation energies, whereas for the acetoxy lactones the activation energies seem to be controlled by the degree of template distortion at the transition state. Finally, calculations show that considering a catalyst on the studied cycloadditions changes the reaction mechanism from concerted to stepwise and proceed with much lower activation energies.
Keywords: DFT calculations; Diels-Alder reactions; activation strain model; charge transfer; chiral anthracenes.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Fringuelli F., Taticchi A., The Diels-Alder Reaction: Selected Practical Methods; Wiley, J. & S., Ed.; John Wiley & Sons.: Baffins Lane, Chichester, 2002.
-
- Houk K. N., Gonzalez J., Li Y., Acc. Chem. Res. 1995, 28 (2), 81–90.
-
- Brocksom T. J., Nakamura J., Ferreira M. L., Brocksom U., Braz J., Chem. Soc. 2001, 12, 597–622.
-
- Ess D. H., Jones G. O., Houk K. N., Adv. Synth. Catal. 2006, 348, 2337–2361.
-
- Funel J. A., Abele S., Angew. Chem. Int. Ed. 2013, 52, 3822–3863; - PubMed
- Angew. Chem. 2013, 125, 3912–3955.
Publication types
LinkOut - more resources
Full Text Sources

