Resistance-Associated Mutations in Chronic Lymphocytic Leukemia Patients Treated With Novel Agents
- PMID: 32670873
- PMCID: PMC7330112
- DOI: 10.3389/fonc.2020.00894
Resistance-Associated Mutations in Chronic Lymphocytic Leukemia Patients Treated With Novel Agents
Abstract
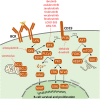
Inhibitors of B-cell receptor signaling, ibrutinib and idelalisib, and BCL-2 antagonist, venetoclax, have become the mainstay of treatment for chronic lymphocytic leukemia (CLL). Despite significant efficacy in most CLL patients, some patients develop resistance to these agents and progress on these drugs. We provide a state-of-the-art overview of the acquired resistance to novel agents. In 80% of patients with ibrutinib failure, acquired mutations in BTK and PLCG2 genes were detected. No distinct unifying resistance-associated mutations or deregulated signaling pathways have been reported in idelalisib failure. Acquired mutations in the BCL2 gene were detected in patients who had failed on venetoclax. In most cases, patients who have progressed on ibrutinib and venetoclax experience resistance-associated mutations, often present at low allelic frequencies. Resistance-associated mutations tend to occur between the second and fourth years of treatment and may already be detected several months before clinical relapse. We also discuss the development of next-generation agents for CLL patients who have acquired resistant mutations to current inhibitors.
Keywords: CLL; genetic aberrations; resistance-associated mutations; targeted therapy; treatment resistance.
Copyright © 2020 Sedlarikova, Petrackova, Papajik, Turcsanyi and Kriegova.
Figures




References
-
- Barr PM, Robak T, Owen C, Tedeschi A, Bairey O, Bartlett NL, et al. Sustained efficacy and detailed clinical follow-up of first-line ibrutinib treatment in older patients with chronic lymphocytic leukemia: extended phase 3 results from RESONATE-2. Haematologica. (2018) 103:1502–10. 10.3324/haematol.2018.192328 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

