Pre-treatment With PLGA/Silibinin Nanoparticles Mitigates Dacarbazine-Induced Hepatotoxicity
- PMID: 32671024
- PMCID: PMC7332747
- DOI: 10.3389/fbioe.2020.00495
Pre-treatment With PLGA/Silibinin Nanoparticles Mitigates Dacarbazine-Induced Hepatotoxicity
Abstract
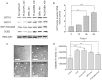
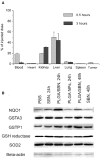
Drug-induced hepatotoxicity is one of the major barriers limiting application of current pharmaceuticals as well as clinical translation of novel and perspective drugs. In this context, numerous hepatoprotective molecules have been proposed to prevent or mitigate drug-induced hepatotoxicity. To date, silibinin (SBN) is a one the most studied hepatoprotective plant-derived agents for prevention/alleviation of drug-induced liver injury. Hepatoprotective mechanisms of SBN include scavenging of free radicals, upregulation of detoxifying enzymes via Nrf2 activation and inhibition of inflammatory activation of resident macrophages. However, low solubility of this phytochemical in water prevents its intravenous administration and constrains its bioavailability and efficacy. Here, we developed SBN-loaded poly(lactic-co-glycolic) acid (PLGA)-based nanoparticles for intravenous administration aiming at mitigation of drug-induced hepatotoxicity. Obtained nanoparticles demonstrated a slow drug release profile in vitro and caused upregulation of antioxidant and phase II enzymes in AML12 hepatocytes including superoxide dismutase 2, glutathione-S-transferase P1, and glutathione-reductase. Intravenous administration of PLGA nanoparticles to mice led to their fast liver accumulation. In vivo analysis of hepatoprotective effects of PLGA/SBN nanoparticles was carried out on melanoma tumor-bearing syngeneic mouse model treated with the antineoplastic drug dacarbazine (DTIC), which often causes severe hepatotoxicity including development of veno-occlusive disease. It was found that PLGA/SBN caused effective induction of detoxifying liver enzymes. Moreover, pre-treatment with PLGA/SBN nanoparticles reduced elevated transaminase and bilirubin levels in blood, caspase 3 activation, and morphological histology changes in liver tissue upon DTIC treatment. Treatment with PLGA/SBN nanoparticles did not interfere with therapeutic efficacy of DTIC.
Keywords: drug combination; drug-induced liver injury; hepatoprotection; melanoma; nanoformulation.
Copyright © 2020 Durymanov, Permyakova and Reineke.
Figures
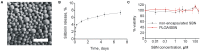



References
LinkOut - more resources
Full Text Sources
Research Materials

