Continuous Cultivation as a Tool Toward the Rational Bioprocess Development With Pichia Pastoris Cell Factory
- PMID: 32671036
- PMCID: PMC7330098
- DOI: 10.3389/fbioe.2020.00632
Continuous Cultivation as a Tool Toward the Rational Bioprocess Development With Pichia Pastoris Cell Factory
Abstract
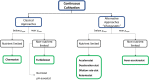
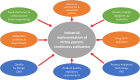
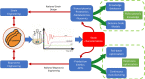
The methylotrophic yeast Pichia pastoris (Komagataella phaffii) is currently considered one of the most promising hosts for recombinant protein production (RPP) and metabolites due to the availability of several tools to efficiently regulate the recombinant expression, its ability to perform eukaryotic post-translational modifications and to secrete the product in the extracellular media. The challenge of improving the bioprocess efficiency can be faced from two main approaches: the strain engineering, which includes enhancements in the recombinant expression regulation as well as overcoming potential cell capacity bottlenecks; and the bioprocess engineering, focused on the development of rational-based efficient operational strategies. Understanding the effect of strain and operational improvements in bioprocess efficiency requires to attain a robust knowledge about the metabolic and physiological changes triggered into the cells. For this purpose, a number of studies have revealed chemostat cultures to provide a robust tool for accurate, reliable, and reproducible bioprocess characterization. It should involve the determination of key specific rates, productivities, and yields for different C and N sources, as well as optimizing media formulation and operating conditions. Furthermore, studies along the different levels of systems biology are usually performed also in chemostat cultures. Transcriptomic, proteomic and metabolic flux analysis, using different techniques like differential target gene expression, protein description and 13C-based metabolic flux analysis, are widely described as valued examples in the literature. In this scenario, the main advantage of a continuous operation relies on the quality of the homogeneous samples obtained under steady-state conditions, where both the metabolic and physiological status of the cells remain unaltered in an all-encompassing picture of the cell environment. This contribution aims to provide the state of the art of the different approaches that allow the design of rational strain and bioprocess engineering improvements in Pichia pastoris toward optimizing bioprocesses based on the results obtained in chemostat cultures. Interestingly, continuous cultivation is also currently emerging as an alternative operational mode in industrial biotechnology for implementing continuous process operations.
Keywords: Pichia pastoris; bioreaction kinetics; continuous cultivation; heterologous protein production; physiological characterization; rational bioprocess development; steady-state omics; systems microbiology.
Copyright © 2020 Nieto-Taype, Garcia-Ortega, Albiol, Montesinos-Seguí and Valero.
Figures

Similar articles
-
Bioprocess performance analysis of novel methanol-independent promoters for recombinant protein production with Pichia pastoris.Microb Cell Fact. 2021 Mar 23;20(1):74. doi: 10.1186/s12934-021-01564-9. Microb Cell Fact. 2021. PMID: 33757505 Free PMC article.
-
Rational development of bioprocess engineering strategies for recombinant protein production in Pichia pastoris (Komagataella phaffii) using the methanol-free GAP promoter. Where do we stand?N Biotechnol. 2019 Nov 25;53:24-34. doi: 10.1016/j.nbt.2019.06.002. Epub 2019 Jun 10. N Biotechnol. 2019. PMID: 31195158 Review.
-
Rationale-based selection of optimal operating strategies and gene dosage impact on recombinant protein production in Komagataella phaffii (Pichia pastoris).Microb Biotechnol. 2020 Mar;13(2):315-327. doi: 10.1111/1751-7915.13498. Epub 2019 Oct 28. Microb Biotechnol. 2020. PMID: 31657146 Free PMC article.
-
Innovative Bioprocess Strategies Combining Physiological Control and Strain Engineering of Pichia pastoris to Improve Recombinant Protein Production.Front Bioeng Biotechnol. 2022 Jan 26;10:818434. doi: 10.3389/fbioe.2022.818434. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35155391 Free PMC article.
-
Second generation Pichia pastoris strain and bioprocess designs.Biotechnol Biofuels Bioprod. 2022 Dec 29;15(1):150. doi: 10.1186/s13068-022-02234-7. Biotechnol Biofuels Bioprod. 2022. PMID: 36581872 Free PMC article. Review.
Cited by
-
Chances and drawbacks of derepressed recombinant enzyme production in continuous cultivations with Komagataella phaffii.Front Bioeng Biotechnol. 2025 Mar 10;13:1523037. doi: 10.3389/fbioe.2025.1523037. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40129455 Free PMC article.
-
β-glucosidase production by recombinant Pichia pastoris strain Y1433 under optimal feed profiles of fed-batch cultivation.Folia Microbiol (Praha). 2023 Apr;68(2):245-256. doi: 10.1007/s12223-022-01008-w. Epub 2022 Oct 14. Folia Microbiol (Praha). 2023. PMID: 36241938
-
Pichia pastoris (Komagataella phaffii) as a Cost-Effective Tool for Vaccine Production for Low- and Middle-Income Countries (LMICs).Bioengineering (Basel). 2021 Aug 31;8(9):119. doi: 10.3390/bioengineering8090119. Bioengineering (Basel). 2021. PMID: 34562941 Free PMC article. Review.
-
Unlocking the power of antimicrobial peptides: advances in production, optimization, and therapeutics.Front Cell Infect Microbiol. 2025 Apr 28;15:1528583. doi: 10.3389/fcimb.2025.1528583. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40365533 Free PMC article. Review.
-
Bioprocess performance analysis of novel methanol-independent promoters for recombinant protein production with Pichia pastoris.Microb Cell Fact. 2021 Mar 23;20(1):74. doi: 10.1186/s12934-021-01564-9. Microb Cell Fact. 2021. PMID: 33757505 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

