Mitophagy, Mitochondrial Homeostasis, and Cell Fate
- PMID: 32671064
- PMCID: PMC7326955
- DOI: 10.3389/fcell.2020.00467
Mitophagy, Mitochondrial Homeostasis, and Cell Fate
Abstract
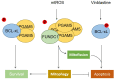
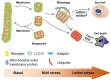
Mitochondria are highly plastic and dynamic organelles that have graded responses to the changing cellular, environmental, and developmental cues. Mitochondria undergo constant mitochondrial fission and fusion, mitochondrial biogenesis, and mitophagy, which coordinately control mitochondrial morphology, quantity, quality, turnover, and inheritance. Mitophagy is a cellular process that selectively removes the aged and damaged mitochondria via the specific sequestration and engulfment of mitochondria for subsequent lysosomal degradation. It plays a pivotal role in reinstating cellular homeostasis in normal physiology and conditions of stress. Damaged mitochondria may either instigate innate immunity through the overproduction of ROS or the release of mtDNA, or trigger cell death through the release of cytochrome c and other apoptogenic factors when mitochondria damage is beyond repair. Distinct molecular machineries and signaling pathways are found to regulate these mitochondrial dynamics and behaviors. It is less clear how mitochondrial behaviors are coordinated at molecular levels. BCL2 family proteins interact within family members to regulate mitochondrial outer membrane permeabilization and apoptosis. They were also described as global regulators of mitochondrial homeostasis and mitochondrial fate through their interaction with distinct partners including Drp1, mitofusins, PGAM5, and even LC3 that involved mitochondrial dynamics and behaviors. In this review, we summarize recent findings on molecular pathways governing mitophagy and its coordination with other mitochondrial behaviors, which together determine cellular fate.
Keywords: cell fate; mitochondrial apoptosis; mitochondrial dynamics; mitophagy; mitophagy receptors.
Copyright © 2020 Ma, Chen, Li, Kepp, Zhu and Chen.
Figures
References
-
- Bell E. L., Klimova T. A., Eisenbart J., Moraes C. T., Murphy M. P., Budinger G. R., et al. (2007). The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J. Cell Biol. 177 1029–1036. 10.1083/jcb.200609074 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

