Identification and sub-cellular localization of a NAD transporter in Leishmania braziliensis (Lb NDT1)
- PMID: 32671255
- PMCID: PMC7350145
- DOI: 10.1016/j.heliyon.2020.e04331
Identification and sub-cellular localization of a NAD transporter in Leishmania braziliensis (Lb NDT1)
Abstract
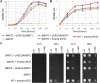
Nicotinamide adenine dinucleotide (NAD) is one of the central molecules involved in energy homeostasis, cellular signaling and antioxidative defense systems. Consequently, its biosynthetic pathways and transport systems are of vital importance. The nicotinamide/nicotinate mononucleotide adenylyltransferase (NMNAT), a key enzyme in the biosynthesis of NAD, is distributed in all domains of life and exhibits various isoforms in free-living organisms in contrast with intracellular parasites, which displays a single enzyme. In Leishmania braziliensis a unique cytosolic NMNAT has been reported to date and the mechanisms through which adequate levels of NAD are maintained among the different sub-cellular compartments of this parasite are unknown. Experimental evidences have related the transport of NAD to the Nucleotide Transporters (NTTs) family, whose members are located in the cytoplasmic membrane of parasitic life organisms. Additionally, the Mitochondrial Carrier Family (MCF), a group of proteins located in the membrane of internal organelles such as the mitochondria of free life organisms, has been implicated in NAD transport. Applying bioinformatics tools, the main characteristics of the MCF were found in a transporter candidate that we have designated as Nicotinamide Adenine Dinucleotide Transporter 1 of L. braziliensis (LbNDT1). The expression of LbNDT1 was tested both in axenic amastigotes and promastigotes of L. braziliensis, through immunodetection using polyclonal avian antibodies produced in this study. N-glycosylation of LbNDT1 was observed in both stages. Additionally, a possible partial mitochondrial distribution for LbNDT1 in amastigotes and a possible glycosomal location in promastigotes are proposed. Finally, the capability of LbNDT1 to transport NAD was confirmed by complementation assays in Saccharomyces cerevisiae. Our results demonstrate the existence of LbNDT1 in L. braziliensis becoming the first NAD transporter identified in protozoan parasites to date.
Keywords: Biochemistry; Bioinformatics; Biomolecules; Cell biology; Energetic metabolism; LbNDT1; Leishmania braziliensis; Molecular biology; NAD transport.
© 2020 The Authors.
Figures





Similar articles
-
Protein-protein interactions of the nicotinamide/nicotinate mononucleotide adenylyltransferase of Leishmania braziliensis.Mem Inst Oswaldo Cruz. 2019 Mar 21;114:e180506. doi: 10.1590/0074-02760180506. Mem Inst Oswaldo Cruz. 2019. PMID: 30916117 Free PMC article.
-
Identification and functional evaluation of Leishmania braziliensis Nicotinamide Mononucleotide Adenylyltransferase.Protein Expr Purif. 2015 Nov;115:26-33. doi: 10.1016/j.pep.2015.08.022. Epub 2015 Aug 28. Protein Expr Purif. 2015. PMID: 26318236
-
Kinetic and oligomeric study of Leishmania braziliensis nicotinate/nicotinamide mononucleotide adenylyltransferase.Heliyon. 2020 Apr 12;6(4):e03733. doi: 10.1016/j.heliyon.2020.e03733. eCollection 2020 Apr. Heliyon. 2020. PMID: 32322725 Free PMC article.
-
The key role of the NAD biosynthetic enzyme nicotinamide mononucleotide adenylyltransferase in regulating cell functions.IUBMB Life. 2022 Jul;74(7):562-572. doi: 10.1002/iub.2584. Epub 2021 Dec 5. IUBMB Life. 2022. PMID: 34866305 Free PMC article. Review.
-
Cellular and Mitochondrial NAD Homeostasis in Health and Disease.Cells. 2023 May 6;12(9):1329. doi: 10.3390/cells12091329. Cells. 2023. PMID: 37174729 Free PMC article. Review.
Cited by
-
N-Glycosylation of MRS2 balances aerobic and anaerobic energy production by reducing rapid mitochondrial Mg2+ influx in conditions of high glucose or impaired respiratory chain function.bioRxiv [Preprint]. 2024 Jul 9:2024.07.09.602756. doi: 10.1101/2024.07.09.602756. bioRxiv. 2024. PMID: 39026824 Free PMC article. Preprint.
References
-
- Agrimi Gennaro, Russo Annamaria, Scarcia Pasquale, Palmieri Ferdinando. The human gene SLC25A17 encodes a peroxisomal transporter of coenzyme A, FAD and NAD+ Biochem. J. 2012;443(1):241–247. - PubMed
-
- Berger Felicitas, Lau Corinna, Dahlmann Mathias, Ziegler Mathias. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J. Biol. Chem. 2005 - PubMed
-
- Blom Nikolaj. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics. 2004;4(6):1633–1649. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

