Progressive dysexecutive syndrome due to Alzheimer's disease: a description of 55 cases and comparison to other phenotypes
- PMID: 32671341
- PMCID: PMC7325839
- DOI: 10.1093/braincomms/fcaa068
Progressive dysexecutive syndrome due to Alzheimer's disease: a description of 55 cases and comparison to other phenotypes
Abstract
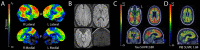
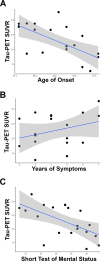
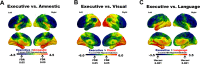
We report a group of patients presenting with a progressive dementia syndrome characterized by predominant dysfunction in core executive functions, relatively young age of onset and positive biomarkers for Alzheimer's pathophysiology. Atypical frontal, dysexecutive/behavioural variants and early-onset variants of Alzheimer's disease have been previously reported, but no diagnostic criteria exist for a progressive dysexecutive syndrome. In this retrospective review, we report on 55 participants diagnosed with a clinically defined progressive dysexecutive syndrome with 18F-fluorodeoxyglucose-positron emission tomography and Alzheimer's disease biomarkers available. Sixty-two per cent of participants were female with a mean of 15.2 years of education. The mean age of reported symptom onset was 53.8 years while the mean age at diagnosis was 57.2 years. Participants and informants commonly referred to initial cognitive symptoms as 'memory problems' but upon further inquiry described problems with core executive functions of working memory, cognitive flexibility and cognitive inhibitory control. Multi-domain cognitive impairment was evident in neuropsychological testing with executive dysfunction most consistently affected. The frontal and parietal regions which overlap with working memory networks consistently demonstrated hypometabolism on positron emission tomography. Genetic testing for autosomal dominant genes was negative in all eight participants tested and at least one APOE ε4 allele was present in 14/26 participants tested. EEG was abnormal in 14/17 cases with 13 described as diffuse slowing. Furthermore, CSF or neuroimaging biomarkers were consistent with Alzheimer's disease pathophysiology, although CSF p-tau was normal in 24% of cases. Fifteen of the executive predominate participants enrolled in research neuroimaging protocols and were compared to amnestic (n = 110), visual (n = 18) and language (n = 7) predominate clinical phenotypes of Alzheimer's disease. This revealed a consistent pattern of hypometabolism in parieto-frontal brain regions supporting executive functions with relative sparing of the medial temporal lobe (versus amnestic phenotype), occipital (versus visual phenotype) and left temporal (versus language phenotype). We propose that this progressive dysexecutive syndrome should be recognized as a distinct clinical phenotype disambiguated from behavioural presentations and not linked specifically to the frontal lobe or a particular anatomic substrate without further study. This clinical presentation can be due to Alzheimer's disease but is likely not specific for any single aetiology. Diagnostic criteria are proposed to facilitate additional research into this understudied clinical presentation.
Keywords: CSF biomarkers; FDG-PET; dysexecutive; early-onset Alzheimer’s disease; tau PET.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures





References
-
- Andreasen N, Minthon L, Davidsson P, Vanmechelen E, Vanderstichele H, Winblad B, et al.Evaluation of CSF-tau and CSF-Abeta42 as diagnostic markers for Alzheimer disease in clinical practice. Arch Neurol 2001; 58: 373–9. - PubMed
-
- Ashburner J, Friston KJ.. Unified segmentation. Neuroimage 2005; 26: 839–51. - PubMed
-
- Baddeley A. Working memory Vol. 11 Oxford: Oxford University Press, Clarendon Press; 1986.
-
- Baddeley A. Working memory. Science 1992; 255: 556–9. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
