Signaling mechanisms of μ-opioid receptor (MOR) in the hippocampus: disinhibition versus astrocytic glutamate regulation
- PMID: 32671427
- PMCID: PMC11073310
- DOI: 10.1007/s00018-020-03595-8
Signaling mechanisms of μ-opioid receptor (MOR) in the hippocampus: disinhibition versus astrocytic glutamate regulation
Abstract
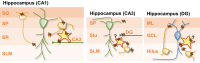
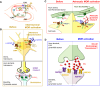
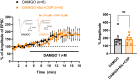
μ-opioid receptor (MOR) is a class of opioid receptors that is critical for analgesia, reward, and euphoria. MOR is distributed in various brain regions, including the hippocampus, where traditionally, it is believed to be localized mainly at the presynaptic terminals of the GABAergic inhibitory interneurons to exert a strong disinhibitory effect on excitatory pyramidal neurons. However, recent intensive research has uncovered the existence of MOR in hippocampal astrocytes, shedding light on how astrocytic MOR participates in opioid signaling via glia-neuron interaction in the hippocampus. Activation of astrocytic MOR has shown to cause glutamate release from hippocampal astrocytes and increase the excitability of presynaptic axon fibers to enhance the release of glutamate at the Schaffer Collateral-CA1 synapses, thereby, intensifying the synaptic strength and plasticity. This novel mechanism involving astrocytic MOR has been shown to participate in hippocampus-dependent conditioned place preference. Furthermore, the signaling of hippocampal MOR, whose action is sexually dimorphic, is engaged in adult neurogenesis, seizure, and stress-induced memory impairment. In this review, we focus on the two profoundly different hippocampal opioid signaling pathways through either GABAergic interneuronal or astrocytic MOR. We further compare and contrast their molecular and cellular mechanisms and their possible roles in opioid-associated conditioned place preference and other hippocampus-dependent behaviors.
Keywords: Astrocyte; Disinhibition; Glutamate; Hippocampus; LTP; μ-opioid receptor.
Figures
Similar articles
-
Activation of Astrocytic μ-Opioid Receptor Causes Conditioned Place Preference.Cell Rep. 2019 Jul 30;28(5):1154-1166.e5. doi: 10.1016/j.celrep.2019.06.071. Cell Rep. 2019. PMID: 31365861
-
Expression of µ-Opioid Receptor in CA1 Hippocampal Astrocytes.Exp Neurobiol. 2018 Apr;27(2):120-128. doi: 10.5607/en.2018.27.2.120. Epub 2018 Apr 24. Exp Neurobiol. 2018. PMID: 29731678 Free PMC article.
-
Computational modeling of opioid-induced synaptic plasticity in hippocampus.PLoS One. 2018 Mar 7;13(3):e0193410. doi: 10.1371/journal.pone.0193410. eCollection 2018. PLoS One. 2018. PMID: 29513763 Free PMC article.
-
Glutamate release from astrocytes as a non-synaptic mechanism for neuronal synchronization in the hippocampus.J Physiol Paris. 2006 Mar-May;99(2-3):98-102. doi: 10.1016/j.jphysparis.2005.12.008. J Physiol Paris. 2006. PMID: 16646155 Review.
-
Direct association of Mu-opioid and NMDA glutamate receptors supports their cross-regulation: molecular implications for opioid tolerance.Curr Drug Abuse Rev. 2012 Sep;5(3):199-226. doi: 10.2174/1874473711205030199. Curr Drug Abuse Rev. 2012. PMID: 22920535 Review.
Cited by
-
Shared Mechanisms of GABAergic and Opioidergic Transmission Regulate Corticolimbic Reward Systems and Cognitive Aspects of Motivational Behaviors.Brain Sci. 2023 May 17;13(5):815. doi: 10.3390/brainsci13050815. Brain Sci. 2023. PMID: 37239287 Free PMC article. Review.
-
Looking to the stars for answers: Strategies for determining how astrocytes influence neuronal activity.Comput Struct Biotechnol J. 2022 Aug 2;20:4146-4156. doi: 10.1016/j.csbj.2022.07.052. eCollection 2022. Comput Struct Biotechnol J. 2022. PMID: 36016711 Free PMC article. Review.
-
Opioidergic pain relief in humans is mediated by beta and high-gamma modulation in limbic regions.medRxiv [Preprint]. 2025 Jun 20:2025.03.03.25323046. doi: 10.1101/2025.03.03.25323046. medRxiv. 2025. PMID: 40093233 Free PMC article. Preprint.
-
Mapping Astrocytic and Neuronal μ-opioid Receptor Expression in Various Brain Regions Using MOR-mCherry Reporter Mouse.Exp Neurobiol. 2023 Dec 31;32(6):395-409. doi: 10.5607/en23039. Exp Neurobiol. 2023. PMID: 38196135 Free PMC article.
-
The Endolysosomal Transporter DMT1 is Required for Morphine Regulation of Neuronal Ferritin Heavy Chain.J Neuroimmune Pharmacol. 2023 Sep;18(3):495-508. doi: 10.1007/s11481-023-10082-x. Epub 2023 Sep 4. J Neuroimmune Pharmacol. 2023. PMID: 37661197 Free PMC article.
References
-
- Stefano GB, Ptacek R, Kuzelova H, Kream RM. Endogenous morphine: up-to-date review 2011. Folia Biol (Praha) 2012;58(2):49–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

