COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases
- PMID: 32671831
- PMCID: PMC7405500
- DOI: 10.1111/cei.13495
COVID-19 vaccine-readiness for anti-CD20-depleting therapy in autoimmune diseases
Abstract
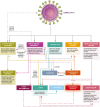
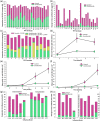
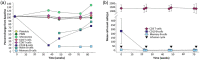
Although most autoimmune diseases are considered to be CD4 T cell- or antibody-mediated, many respond to CD20-depleting antibodies that have limited influence on CD4 and plasma cells. This includes rituximab, oblinutuzumab and ofatumumab that are used in cancer, rheumatoid arthritis and off-label in a large number of other autoimmunities and ocrelizumab in multiple sclerosis. Recently, the COVID-19 pandemic created concerns about immunosuppression in autoimmunity, leading to cessation or a delay in immunotherapy treatments. However, based on the known and emerging biology of autoimmunity and COVID-19, it was hypothesised that while B cell depletion should not necessarily expose people to severe SARS-CoV-2-related issues, it may inhibit protective immunity following infection and vaccination. As such, drug-induced B cell subset inhibition, that controls at least some autoimmunities, would not influence innate and CD8 T cell responses, which are central to SARS-CoV-2 elimination, nor the hypercoagulation and innate inflammation causing severe morbidity. This is supported clinically, as the majority of SARS-CoV-2-infected, CD20-depleted people with autoimmunity have recovered. However, protective neutralizing antibody and vaccination responses are predicted to be blunted until naive B cells repopulate, based on B cell repopulation kinetics and vaccination responses, from published rituximab and unpublished ocrelizumab (NCT00676715, NCT02545868) trial data, shown here. This suggests that it may be possible to undertake dose interruption to maintain inflammatory disease control, while allowing effective vaccination against SARS-CoV-29, if and when an effective vaccine is available.
Keywords: B cell; CD20; COVID-19; autoimmunity; immunotherapy; multiple sclerosis; ocrelizumab; rheumatoid arthritis; rituximab.
© 2020 The Authors. Clinical & Experimental Immunology published by John Wiley & Sons Ltd on behalf of British Society for Immunology.
Conflict of interest statement
D. B., M. M., K. S. and G. G. have received compensation for either consultancies and presentations and advisory board activities from Genentech/Roche. However, Roche/Genentech were not involved in the decision to write and submit this manuscript. S. A. has received consultancy from Novartis and Roche. C. A. K. K., G. P., A. S. K. and S. R. have nothing to disclose. D. B. has received compensation for activities related to Canbex therapeutics, InMune Biol, Lundbeck, Japan tobacco, Merck and Novartis. M. M. has received speaking honoraria from Sanofi‐Genzyme. K. S. has received compensation for activities related to Biogen, Eisai, Elan, Fiveprime, Lipomed, Merck KGAa, Novartis, Sanofi‐Genzyme and Teva. G. G. has received compensation for activities from AbbVie, Actelion, Atara Bio, Bayer‐Schering Healthcare, Biogen, Celgene, GW Pharma, GSK, Ironwood, Japanese Tobacco, Merck, Merck‐Serono, Mertz, Novartis, Pfizer, Sanofi‐Genzyme, Synthon, Takeda, Teva, UCB Pharma and Vertex Pharmaceuticals.
Figures
References
-
- Baker D, Pryce G, Amor S, Giovannoni G, Schmierer K. Learning from other autoimmunities to understand targeting of B‐cells to control multiple sclerosis. Brain 2018; 141:2824–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

