Antibiotic adjuvant therapy for pulmonary infection in cystic fibrosis
- PMID: 32671834
- PMCID: PMC8407502
- DOI: 10.1002/14651858.CD008037.pub4
Antibiotic adjuvant therapy for pulmonary infection in cystic fibrosis
Abstract
Background: Cystic fibrosis is a multi-system disease characterised by the production of thick secretions causing recurrent pulmonary infection, often with unusual bacteria. This leads to lung destruction and eventually death through respiratory failure. There are no antibiotics in development that exert a new mode of action and many of the current antibiotics are ineffective in eradicating the bacteria once chronic infection is established. Antibiotic adjuvants - therapies that act by rendering the organism more susceptible to attack by antibiotics or the host immune system, by rendering it less virulent or killing it by other means, would be a significant therapeutic advance. This is an update of a previously published review.
Objectives: To determine if antibiotic adjuvants improve clinical and microbiological outcome of pulmonary infection in people with cystic fibrosis.
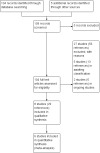
Search methods: We searched the Cystic Fibrosis Trials Register which is compiled from database searches, hand searches of appropriate journals and conference proceedings. Date of most recent search: 16 January 2020. We also searched MEDLINE (all years) on 14 February 2019 and ongoing trials registers on 06 April 2020.
Selection criteria: Randomised controlled trials and quasi-randomised controlled trials of a therapy exerting an antibiotic adjuvant mechanism of action compared to placebo or no therapy for people with cystic fibrosis.
Data collection and analysis: Two of the authors independently assessed and extracted data from identified trials.
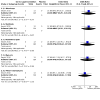
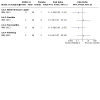
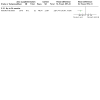
Main results: We identified 42 trials of which eight (350 participants) that examined antibiotic adjuvant therapies are included. Two further trials are ongoing and five are awaiting classification. The included trials assessed β-carotene (one trial, 24 participants), garlic (one trial, 34 participants), KB001-A (a monoclonal antibody) (two trials, 196 participants), nitric oxide (two trials, 30 participants) and zinc supplementation (two trials, 66 participants). The zinc trials recruited children only, whereas the remaining trials recruited both adults and children. Three trials were located in Europe, one in Asia and four in the USA. Three of the interventions measured our primary outcome of pulmonary exacerbations (β-carotene, mean difference (MD) -8.00 (95% confidence interval (CI) -18.78 to 2.78); KB001-A, risk ratio (RR) 0.25 (95% CI 0.03 to 2.40); zinc supplementation, RR 1.85 (95% CI 0.65 to 5.26). β-carotene and KB001-A may make little or no difference to the number of exacerbations experienced (low-quality evidence); whereas, given the moderate-quality evidence we found that zinc probably makes no difference to this outcome. Respiratory function was measured in all of the included trials. β-carotene and nitric oxide may make little or no difference to forced expiratory volume in one second (FEV1) (low-quality evidence), whilst garlic probably makes little or no difference to FEV1 (moderate-quality evidence). It is uncertain whether zinc or KB001-A improve FEV1 as the certainty of this evidence is very low. Few adverse events were seen across all of the different interventions and the adverse events that were reported were mild or not treatment-related (quality of the evidence ranged from very low to moderate). One of the trials (169 participants) comparing KB001-A and placebo, reported on the time to the next course of antibiotics; results showed there is probably no difference between groups, HR 1.00 (95% CI 0.69 to 1.45) (moderate-quality evidence). Quality of life was only reported in the two KB001-A trials, which demonstrated that there may be little or no difference between KB001-A and placebo (low-quality evidence). Sputum microbiology was measured and reported for the trials of KB001-A and nitric oxide (four trials). There was very low-quality evidence of a numerical reduction in Pseudomonas aeruginosa density with KB001-A, but it was not significant. The two trials looking at the effects of nitric oxide reported significant reductions in Staphylococcus aureus and near-significant reductions in Pseudomonas aeruginosa, but the quality of this evidence is again very low.
Authors' conclusions: We could not identify an antibiotic adjuvant therapy that we could recommend for treating of lung infection in people with cystic fibrosis. The emergence of increasingly resistant bacteria makes the reliance on antibiotics alone challenging for cystic fibrosis teams. There is a need to explore alternative strategies, such as the use of adjuvant therapies. Further research is required to provide future therapeutic options.
پیشینه: فیبروز سیستیک یک بیماری چند سیستمی است که مشخصه آن، تولید ترشحات ضخیمی است که باعث عفونت مکرر ریوی، غالبا با باکتریهای غیرمعمول میشود. این وضعیت منجر به تخریب ریه و در نهایت مرگ در اثر نارسایی تنفسی میشود. هیچ آنتیبیوتیک در حال توسعهای وجود ندارد که دارای یک روش جدید عملکرد باشد و بسیاری از آنتیبیوتیکهای فعلی، در ریشهکن کردن باکتریها پس از استقرار عفونت مزمن بیاثر هستند. داروهای کمک کننده به آنتیبیوتیک ‐ درمانهایی که ارگانیسم را با حساستر کردن آن به حمله آنتیبیوتیکها یا سیستم ایمنی بدن میزبان، یا با کاهش بیماریزایی آن یا کشتن آن با روشهای دیگر تسلیم میکند، پیشرفت درمانی قابلتوجهی خواهد بود. این یک بهروزرسانی از مرور منتشر شده قبلی است. اهداف: تعیین اینکه داروهای کمک کننده به آنتیبیوتیک باعث بهبود پیامد بالینی و میکروبیولوژیکی عفونت ریوی در افراد مبتلا به فیبروز سیستیک میشوند یا خیر. روشهای جستوجو: ما پایگاه ثبت کارآزماییهای گروه فیبروز سیستیک در کاکرین را جستوجو کردیم که شامل جستوجوها در بانکهای اطلاعاتی و جستوجوی دستی در مجلات و کتاب چکیده مقالات کنفرانسهای مرتبط بود. تاریخ آخرین جستوجو: 16 ژانویه 2020. ما همچنین MEDLINE (همه سالها) را در 14 فوریه 2019 و پایگاههای ثبت کارآزماییهای در حال انجام را در 6 اپریل 2020 جستوجو کردیم. معیارهای انتخاب: کارآزماییهای تصادفیسازی و کنترل شده و کارآزماییهای شبه‐تصادفیسازی و کنترل شده حاوی استفاده از یک درمان با مکانیسم عملکرد کمک به آنتیبیوتیک، در مقایسه با دارونما (placebo) یا عدم درمان برای مبتلایان به فیبروز سیستیک. گردآوری و تجزیهوتحلیل دادهها: دو نفر از نویسندگان بهطور مستقل از هم، دادههای به دست آمده از کارآزماییهای شناسایی شده را ارزیابی و استخراج کردند. نتایج اصلی: ما 42 کارآزمایی را شناسایی کردیم که از این میان، هشت (350 شرکتکننده) کارآزمایی وارد شدند که درمانهای کمک کننده به آنتیبیوتیک را مورد بررسی قرار دادند. دو کارآزمایی دیگر در حال انجام و پنج مورد در انتظار طبقهبندی هستند. کارآزماییهای وارد شده به بررسی β‐ کاروتن (یک کارآزمایی، 24 شرکتکننده)، سیر (یک کارآزمایی، 34 شرکتکننده)، KB001‐A (یک آنتیبادی مونوکلونال) (دو کارآزمایی، 196 شرکتکننده)، نیتریک اکسید (دو کارآزمایی، 30 شرکتکننده) و مکمل زینک (دو کارآزمایی، 66 شرکتکننده) پرداختند. کارآزماییهای زینک فقط روی کودکان انجام شدند، در حالی که کارآزماییهای باقیمانده هم بزرگسالان و هم کودکان را جذب کردند. سه کارآزمایی در اروپا، یک مورد در آسیا و چهار مورد در ایالات متحده انجام شدند. سه مورد از مداخلات، پیامد اولیه ما را که تشدیدهای حملات ریوی بودند، اندازهگیری کردند (β‐کاروتن، تفاوت میانگین (MD): 8.00‐ (95% فاصله اطمینان (CI): 18.78‐ تا 2.78)؛ KB001‐A، خطر نسبی (RR): 0.25؛ (95% CI؛ 0.03 تا 2.40)؛ مکمل زینک، RR؛ 1.85 (95% CI؛ 0.65 تا 5.26). β‐کاروتن و KB001‐A ممکن است تفاوتی اندک یا عدم تفاوت در تعداد تشدیدهای حملات ریوی ایجاد کنند (شواهد با کیفیت پائین)؛ در حالیکه با توجه به شواهدی با کیفیت متوسط، دریافتیم که زینک احتمالا هیچ تفاوتی را روی این پیامد ایجاد نمیکند. عملکرد تنفسی در تمامی کارآزماییهای وارد شده اندازهگیری شد. β‐کاروتن و نیتریک اکسید ممکن است تفاوتی اندک یا عدم تفاوت در حجم اجباری بازدمی در یک ثانیه (FEV1) ایجاد کند (شواهد با کیفیت پایین)، در حالی که سیر احتمالا تفاوتی اندک یا عدم تفاوت روی FEV1 خواهد داشت (شواهد با کیفیت متوسط). مشخص نیست که زینک یا KB001‐A باعث بهبود FEV1 میشوند یا خیر، زیرا قطعیت این شواهد بسیار پائین است. تعداد اندکی از عوارض جانبی در طول تمامی مداخلات مختلف مشاهده شد و عوارض جانبی که گزارش شدند، خفیف بوده یا ارتباطی با درمان نداشتند (کیفیت شواهد از خیلی پایین تا متوسط). یکی از کارآزماییها (169 شرکتکننده) با مقایسه KB001‐A و دارونما، زمان سپری شده را تا نیاز به دوره بعدی درمان با آنتیبیوتیکها گزارش کرد؛ نتایج نشان داد که احتمالا هیچ تفاوتی بین گروهها وجود ندارد، HR؛ 1.00 (95% CI؛ 0.69 تا 1.45) (شواهد با کیفیت متوسط). کیفیت زندگی فقط در دو کارآزمایی KB001‐A گزارش شد، که نشان داد ممکن است تفاوتی اندک یا عدم تفاوت بین KB001‐A و دارونما وجود داشته باشد (شواهد با کیفیت پایین). میکروبیولوژی خلط در کارآزماییهای KB001‐A و اکسید نیتریک (چهار کارآزمایی) اندازهگیری و گزارش شد. شواهدی با کیفیت بسیار پائین در مورد کاهش عددی در تراکم سودوموناس آئروژینوزا با KB001‐A وجود داشت، اما معنیدار نبود. دو کارآزمایی که به بررسی اثرات نیتریک اکسید پرداختند، از کاهش قابل توجه در استافیلوکوکوس اورئوس (Staphylococcus aureus) و تقریبا قابل توجه در سودوموناس آئروژینوزا خبر دادند، اما کیفیت این شواهد دوباره بسیار پایین است. نتیجهگیریهای نویسندگان: ما نتوانستیم یک درمان کمک کننده را به آنتیبیوتیک شناسایی کنیم که بتوانیم آن را برای درمان عفونت ریه در مبتلایان به فیبروز سیستیک پیشنهاد دهیم. ظهور باکتریهایی که بهطور فزایندهای مقاوم هستند، اتکای تنها به آنتیبیوتیکها را برای تیمهای فیبروز سیستیک به چالش میکشد. نیاز به کشف استراتژیهای جایگزین، مانند استفاده از روشهای درمانی کمکی وجود دارد. انجام تحقیقات بیشتر برای ارائه گزینههای درمانی آینده مورد نیاز است.
Trial registration: ClinicalTrials.gov NCT01983774 NCT00633191 NCT00742092 NCT02453789 NCT00928135 NCT02157922.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Figures


























Update of
-
Antibiotic adjuvant therapy for pulmonary infection in cystic fibrosis.Cochrane Database Syst Rev. 2013 Jun 5;2013(6):CD008037. doi: 10.1002/14651858.CD008037.pub3. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2020 Jul 16;7:CD008037. doi: 10.1002/14651858.CD008037.pub4. PMID: 23737089 Free PMC article. Updated.
References
References to studies included in this review
Abdulhamid 2008 {published and unpublished data}
-
- Abdulhamid I, Millard S, Beck F, Chen X, Van Wagnen C, Prasad A. Effect of zinc supplementation on respiratory tract infections in children with cystic fibrosis. Pediatric Pulmonology 2005;40 Suppl 28:348. - PubMed
Howlin 2017 {published data only}
-
- Cathie K, Howlin R, Barraud N, Carroll M, Clarke S, Connett G, et al. Low dose nitric oxide as adjunctive therapy to reduce antimicrobial tolerance of pseudomonas aeruginosa biofilms in the treatment of patients with cystic fibrosis: Report of a proof of concept clinical trial. American Journal of Respiratory and Critical Care Medicine 2014;189:A2843.
-
- Cathie K, Howlin R, Carroll M, Clarke S, Connett G, Cornelius V, et al. RATNO-reducing antibiotic tolerance using nitric oxide in cystic fibrosis: Report of a proof of concept clinical trial. Archives of disease in childhood 2014;99:A159.
Jain 2018 {published data only}
-
- Chmiel J, Hamblett NM, Geller D, Konstan M, VanDevanter D, Thompson V, et al. A phase 2, 16-week, randomized, double-blind, placebo-controlled study to assess safety, tolerability and efficacy of repeated doses of KB001-A in subjects infected with P. aeruginosa. Pediatric Pulmonology 2015;50 Suppl 41:272. [ABSTRACT NO.: 218] [CENTRAL: 1092183] [CFGD REGISTER: PI287]
-
- Jain R, Beckett VV, Konstan MW, Accurso FJ, Burns JL, Mayer-Hamblett N, et al. KB001-A, a novel anti-inflammatory, found to be safe and well-tolerated in cystic fibrosis patients infected with Pseudomonas aeruginosa. Journal of Cystic Fibrosis 2018;17(4):484-91. - PubMed
-
- NCT01695343. Study to evaluate the effect of KB001-A on time-to-need for antibiotic treatment (KB001-A). www.clinicaltrials.gov/ct2/show/NCT01695343 (first received 27 September 2012).
Milla 2013 {published and unpublished data}NCT00638365
-
- Milla C, Accurso F, Chmiel J, McCoy K, Billings J, Atkinson J, et al. A phase I/II study of the anti-PcrV antibody KB001 in cystic fibrosis patients with pseudomonas aeruginosa. American Journal of Respiratory and Critical Care Medicine 2009;179:A1190. [CFGD REGISTER: PI267d]
-
- Milla C, Chmiel J, McCoy KS, Accurso FJ, Billings J, Boyle MP, et al. A phase 1/2 randomized, double-blind, placebo-controlled, single-dose, dose escalation study of KB001 in cystic fibrosis patients infected with pseudomonas aeruginosa. Pediatric Pulmonology 2008;43 Suppl 31:341. [ABSTRACT NO.: 392] [CENTRAL: 921645] [CFGD REGISTER: PI267b ]
-
- Milla C, Chmiel JF, Accurso FJ, McCoy KS, Billings JL, Atkinson JJ, et al. Anti-inflammatory effect of KB001, an anti-PCRV antibody fragment, in CF patients chronically infected with pseudomonas aeruginosa. Pediatric Pulmonology 2009;44 Suppl 32:341. [ABSTRACT NO.: 369] [CENTRAL: 921646] [CFGD REGISTER: PI267c ]
-
- Milla CE, Accurso F J, Chmiel J, McCoy K, Billings J, Atkinson JJ, et al. Modulating Pseudomonas aeruginosa chronic Inflammation with the anti-PcrV antibody KB001: Results of a pilot clinical and pharmacodynamic study In subjects with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A1845. [CENTRAL: 758759] [CFGD REGISTER: PI267g]
-
- Milla CE, Chmiel JF, Accurso FJ, Vandevanter DR, Konstan MW, Yarranton G, et al. Anti-PcrV antibody in cystic fibrosis: a novel approach targeting Pseudomonas aeruginosa airway infection. Pediatric Pulmonology 2014;49(7):650-8. [CENTRAL: 995342] [CFGD REGISTER: PI267e] [EMBASE: 2014431697] - PMC - PubMed
Renner 2001 {published data only}
-
- Engl B, Rust P, Eichler I, Renner S, Elmadfa I. Bioavailability of therapeutic Beta-Carotine (BC) in patients with cystic fibrosis (CF) and effects on anthropometrical parameters over 6 months. Monatsschrift fur Kinderheilkunde 1997;145:S 134. [CFGD REGISTER: GN69g]
-
- Renner S, Wojnarowski C, Koller DY, Rust P, Elmadfa I, Eichler I. Patients with cystic fibrosis (CF) benefit from β-Carotene supplementation for 6 months. In: 22nd European Cystic Fibrosis Conference; 1998 June 13-19; Berlin, Germany. 1998:97. [CFGD REGISTER: GN69b]
-
- Renner S, Wojnarowski C, Koller DY, Rust P, Elmadfa I, Eichler I. Patients with cystic fibrosis (CF) benefit from β-Carotene supplementation for 6 months. Pediatric Pulmonology 1997;24(Suppl 15):314. [CFGD REGISTER: GN69a]
-
- Rust P, Eichler I, Elmadfa I. Influence of an oral beta-carotene supplementation on the antioxidant status of patients with cystic fibrosis. Atemwegs und Lungenkrankheiten 1997;23:51. [CFGD REGISTER: GN69a]
Sagel 2009 {published data only}
-
- NCT00570349. Safety and Tolerability of Inhaled Nitric Oxide in Patients with Cystic Fibrosis. www.clinicaltrials.gov/ct2/show/NCT00570349 (first received 10 December 2007).
-
- Sagel SD, Monchil L, Parker D, Emmett P, Wagner B, Abman S. Safety and antimicrobial effects of inhaled nitric oxide in CF: a pilot study. Pediatric Pulmonology 2009;44 Suppl 32:299. [ABSTRACT NO.: 251] [CENTRAL: 921940] [CFGD REGISTER: OV21]
Sharma 2016 {published data only}
-
- Kabra SK. Effect of zinc supplementation in children with cystic fibrosis. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=3508&EncHid=&us... (first received 14 December 2011).
-
- Sharma G, Lodha R, Shastri S, Saini S, Kapil A, Singla M, et al. Zinc supplementation for one year among children with cystic fibrosis does not decrease pulmonary infection. Respiratory Care 2016;61(1):78-84. [CFGD REGISTER: GN256] [PMID: ] - PubMed
Smyth 2010 {published data only}
-
- Smyth A, Cifelli P, Lewis S, Erskine P, Holland E, Willaims P, et al. Quorum sensing molecules in sputum and plasma as biomarkers in patients with CF and chronic pseudomonas aeruginosa. Pediatric Pulmonology 2008;43(Suppl 31):337. [CFGD REGISTER: PI238c]
-
- Smyth A, Cifelli P, Lewis S, Erskine P, Holland E, Williams P, et al. A randomized controlled trial of macerated garlic oil in patients with CF and chronic pseudomonas aeruginosa. Pediatric Pulmonology 2008;43(Suppl 31):336. [CFGD REGISTER: PI238b]
-
- Smyth A. The Garlic Against Pseudomonas (GAP) study [A randomised controlled trial (pilot study) of the use of macerated garlic in patients with cystic fibrosis who have pulmonary infection with Pseudomonas aeruginosa]. www.isrctn.com/ISRCTN21133397 (first received 01 April 2007). [CFGD REGISTER: PI238a] [DOI: 10.1186/ISRCTN21133397] - DOI
-
- Smyth AR, Cifelli PM, Ortori CA, Righetti K, Lewis S, Erskine P, et al. Garlic as an inhibitor of Pseudomonas aeruginosa quorum sensing in cystic fibrosis – a pilot randomised controlled trial. Pediatric Pulmonology 2010;45(4):356-62. [CFGD REGISTER: PI238d] - PubMed
References to studies excluded from this review
Alvarez 2017 {published data only}
-
- Alvarez JA, Chong EY, Walker DI, Chandler JD, Michalski ES, Grossmann RE, et al. Corrigendum to "Plasma metabolomics in adults with cystic fibrosis during a pulmonary exacerbation: a pilot randomized study of high-dose vitamin D3 administration". Metabolism: Clinical and Experimental 2017;74:41-2. [CFGD REGISTER: GN217h] - PubMed
-
- Alvarez JA, Chong EY, Walker DI, Chandler JD, Michalski ES, Grossmann RE, et al. Plasma metabolomics in adults with cystic fibrosis during a pulmonary exacerbation: a pilot randomized study of high-dose vitamin D3 administration. Metabolism: Clinical and Experimental 2017;70:31-41. [CFGD REGISTER: GN217f] - PMC - PubMed
-
- Alvarez JA, Smith EM, Jones DP, Grossman RE, Frediani JK, Uppal K, et al. Discovery-based, high resolution plasma metabolomics following a vitamin D3 intervention in adult patients with cystic fibrosis. Pediatric Pulmonology 2014;49 Suppl 38:418-9. [ABSTRACT NO.: 551] [CFGD REGISTER: GN217g]
-
- Grossmann RE, Zughaier S, Kumari M, Seydafkan S, Liu S, Lyles R, et al. Clinical responses to a novel vitamin D supplementation strategy in adult CF patients hospitalized for pulmonary exacerbations. Pediatric Pulmonology 2011;46 Suppl 34:404. [ABSTRACT NO.: 526] [CFGD REGISTER: GN217b]
Brown 1985 {published data only}
-
- Brown J, Mellis CM, Wood RE. EDTA aerosol in pseudomonal lung infection. In: 9th International Cystic Fibrosis Congress; 1984 June 9-15; Brighton, England. 1984:4.10. [CFGD REGISTER: PI235a]
-
- Brown J, Mellis CM, Wood RE. Edetate sodium aerosol in Pseudomonas lung infection in cystic fibrosis. American Journal of Diseases of Children 1985;139(8):836-9. [CFGD REGISTER: PI235b] - PubMed
DiMango 2014 {published data only}
-
- DiMango E, Walker P, Keating C, Berdella M, Robinson B, Langfelder-Schwind E, et al. Effect of proton pump inhibitors on cystic fibrosis (CF) pulmonary exacerbations in adults [abstract]. Pediatric Pulmonology 2012;47 Suppl 35:353, Abstract no: 362. [CENTRAL: 962121] [CFGD REGISTER: GN235a]
-
- Dimango E, Walker P, Keating C, Berdella M, Robinson N, Langfelder-Schwind E, et al. Effect of esomeprazole versus placebo on pulmonary exacerbations in cystic fibrosis. BMC Pulmonary Medicine 2014;14:21. [CFGD REGISTER: GN235b] [CTG: ] [GR: P30ES009089/ES/NIEHS NIH HHS/United States] [JID: 100968563] [PMCID: PMC3931289] [PMID: ] [UL1: TR000040/TR/NCATS NIH HHS/United States] - PMC - PubMed
Durairaj 2007 {published data only}
-
- Durairaj L, Launspach J, Watt JL, Mohamad Z, Kline J, Zabner J. Safety assessment of inhaled xylitol in subjects with cystic fibrosis. Journal of Cystic Fibrosis 2007 Jan;6(1):31-4. - PubMed
Forrester 2015 {published data only}ISRCTN22534872
-
- Forrester D, Knox A, Smyth A, Barr H, Simms R, Pacey S, et al. Glutamine supplementation for cystic fibrosis - a randomised controlled trial. Pediatric Pulmonology 2011;46 Suppl 34:288. [ABSTRACT NO.: 214] [CENTRAL: 867950] [CFGD REGISTER: GN231a] - PubMed
Gontijo‐Amaral 2012 {published data only}
-
- Gontijo-Amaral C, Guimarães EV, Camargos P. Oral magnesium supplementation in children with cystic fibrosis improves clinical and functional variables: a double-blind, randomized, placebo-controlled crossover trial. American Journal of Clinical Nutrition 2012;96(1):50-6. [CENTRAL: 880141] [CFGD REGISTER: GN241] [DOI: 10.3945/ajcn.112.034207] [PMID: ] - DOI - PubMed
Grasemann 2013 {published data only}
-
- Grasemann H, Tullis E, Ratjen F. A randomized placebo controlled study on the effects of L-arginine inhalation in patients with cystic fibrosis. Pediatric Pulmonology 2011;46 Suppl 34:293. [ABSTRACT NO.: 229] [CENTRAL: 921615] [CFGD REGISTER: GN233a] [DOI: 10.1016/j.jcf.2012.12.008] - DOI
-
- Grasemann H, Tullis E, Ratjen F. Inhaled L-arginine in patients with cystic fibrosis - a randomized controlled trial. Journal of Cystic Fibrosis 2011;10 Suppl 1:S21. [ABSTRACT NO.: 81] [CENTRAL: 871494] [CFGD REGISTER: GN233b] - PubMed
Hauber 2008 {published data only}
-
- Pforte A, Hauber H P, Mack D, Schumacher U. Inhalation with Fucose and Galactose a new therapeutic approach in adult cystic fibrosis patients with Pseudomonas aeruginosa-Infection [Inhalation mit Fucose und Galactose als neuer Therapieansatz bei erwachsenen Mukoviszidosepatienten mit Pseudomonas aeruginosa-Infektion]. Pneumologie (Stuttgart, Germany) 2001;55(SH1):S68. [CENTRAL: 402236] [CFGD REGISTER: PI268b]
Hodges 2014 {published data only}
-
- Hodges L, MacGregor G, Stevens H, Dessen A, Myrset A. An open label, randomised, two-way crossover scintigraphic study to investigate lung deposition of radiolabelled alginate oligosaccharide delivered as a dry powder and as a nebulised solution in cystic fibrosis patients. Pediatric Pulmonology 2014;49 Suppl 38:305. [ABSTRACT NO.: 251] [CENTRAL: 1012384] [CFGD REGISTER: BD215]
Homnick 1995 {published data only}
-
- Homnick DN, Spillers CR, Cox SR, Cox JH, Yelton LA, DeLoof MJ, et al. Single- and multiple-dose-response relationships of beta-carotene in cystic fibrosis [see comment]. Journal of Pediatrics 1995;127(3):491-4. [CFGD REGISTER: GN68] - PubMed
Kollberg 2003 {published data only}
-
- Kollberg H, Carlander D, Olesen H, Wejaker PE, Johannesson M, Larsson A. Oral administration of specific yolk antibodies (IgY) may prevent Pseudomonas aeruginosa infections in patients with cystic fibrosis: a phase I feasibility study. Pediatric Pulmonology 2003;35(6):433-40. - PubMed
-
- Nilsson E, Kollberg H, Johannesson M, Wejaker PE, Carlander D, Larsson A. More than 10 years' continuous oral treatment with specific immunoglobulin Y for the prevention of Pseudomonas aeruginosa infections: a case report. Journal of Medicinal Food 2007;10(2):375-8. - PubMed
-
- Nilsson E, Larsson A, Olesen HV, Wejaker PE, Kollberg H. Good effect of IgY against Pseudomonas aeruginosa infections in cystic fibrosis patients. Pediatric Pulmonology 2008;43(9):892-9. - PubMed
Kollberg 2010 {published data only}
-
- Kollberg H, Larsson A, Nilsson E. Anti-pseudomonas IGY ready for phase III. Pediatric Pulmonology 2010;45(Suppl 33):343. [ABSTRACT NO.: 345] [CFGD REGISTER: PI252a]
-
- Larsson A. Phase III study (IMPACTT) on anti-pseudomonas IgY. Journal of Cystic Fibrosis 2011;10(Suppl 1):S24. [ABSTRACT NO.: 92] [CFGD REGISTER: PI252b]
-
- Schuster A, Bend J, Hoiby N, Verde PE, Rottmann A, Larsson A, et al. Clinical study to evaluate an anti-Pseudomonas aeruginosa IgY gargling solution (EUDRACT 2011-000801-39). Journal of Cystic Fibrosis 2019;18:S23. [ABSTRACT NO.: WS12-5] [CENTRAL: CN-01990653] [CFGD REGISTER: PI252d] [EMBASE: 2001976053]
Kutateladze 2008 {published data only}
-
- Kutateladze M, Adamia R. Phage therapy experience at the Eliava Institute. Medecine et Maladies Infectieuses 2008;38(8):426-30. - PubMed
Lands 2010 {published data only}
Leonard 2012 {published data only}NCT00945347
-
- Leonard A, Dingemanse J, Lebecque P, Leal T. Oral miglustat in homozygous F508del CF patients. Journal of Cystic Fibrosis 2010;9 Suppl 1:S20. [ABSTRACT NO.: 75] [CFGD REGISTER: BD193a]
Middleton 2015 {published data only}
-
- Middleton A, Robinson P, McKay K, Selvadurai H. Pilot study of inhaled dry powder mannitol in young people with CF hospitalised with pulmonary exacerbation. Pediatric Pulmonology 2012;47 Suppl 35:355. [ABSTRACT NO.: 368] [CENTRAL: 962113] [CFGD REGISTER: BD199a]
-
- Middleton A, Robinson P, McKay K, Selvadurai H. Pilot study of inhaled dry powder mannitol in young people with cystic fibrosis hospitalised with pulmonary exacerbation. Department of Respiratory Medicine, Children's Hospital at Westmead (www.thoracic.org.au) (accessed 21 Oct 2014) 2014. [CENTRAL: 1012538] [CFGD REGISTER: BD199b]
-
- Middleton A, Robinson PD, McKay K, Jaffe A, Selvadurai H. A pilot study of inhaled dry-powder mannitol during cystic fibrosis-related pulmonary exacerbation [letter]. European Respiratory Journal 2015;45(2):541-4. [CENTRAL: 1048444] [CFGD REGISTER: BD199c] [EMBASE: 2015713766] - PubMed
Moss 2013 {published data only}
-
- Moss RB, Mistry SJ, Konstan MW, Pilewski JM, Kerem E, Tal-Singer R, et al. Online Supplement to: "Safety and early treatment effects of the CXCR2 antagonist SB-656933 in patients with cystic fibrosis". Journal of Cystic Fibrosis 2013;13(3):241-8. [CENTRAL: 1000130] [CFGD REGISTER: PI275b] [NCT00903201] - PubMed
-
- Moss RB, Mistry SJ, Konstan MW, Pilewski JM, Kerem E, Tal-Singer R, et al. Safety and early treatment effects of the CXCR2 antagonist SB-656933 in patients with cystic fibrosis. Journal of Cystic Fibrosis 2013;12(3):241-8. [CENTRAL: 967627] [CFGD REGISTER: PI275a] [JID: 101128966] [PMID: ] [SI: ClinicalTrials.gov/NCT00903201] - PubMed
NCT00742092 {unpublished data only}
-
- NCT00742092. Single center, double-blind, randomized, placebo-controlled, two-period/two-treatment crossover study investigating the effect of miglustat on the nasal potential difference in patients with cystic fibrosis homozygous for the F508del mutation. clinicaltrials.gov/ct2/show/NCT00742092 (first received 27 August 2008).
NCT01455675 {published data only}NCT01455675
-
- NCT01455675. Phase III study to evaluate clinical efficacy and safety of avian polyclonal anti-Pseudomonas antibodies (IgY) in prevention of recurrence of Pseudomonas aeruginosa infection in cystic fibrosis patients. clinicaltrials.gov/ct2/show/NCT01455675 (first received 20 October 2011).
Olveira 2010 {published data only}
-
- Olveira G, Olveira C, Acosta E, Espíldora F, Garrido-Sánchez L, García-Escobar E. Fatty acid supplements improve respiratory, inflammatory and nutritional parameters in adults with cystic fibrosis. Archivos de Bronconeumologia 2010;46(2):70-7. - PubMed
Panchaud 2006 {published data only}
-
- Panchaud A, Sauty A, Kernen Y, Decosterd LA, Buclin T, Boulat O, et al. Biological effects of a dietary omega-3 polyunsaturated fatty acids supplementation in cystic fibrosis patients: a randomized, crossover placebo-controlled trial. Clinical Nutrition 2006;25(3):418-27. [CFGD REGISTER: GN109b] [DOI: 10.1016/j.clnu.2005.10.011] - DOI - PubMed
-
- Panchaud A, Sauty A, Kernen Y, Decosterd LA, Buclin T, Roule M. Dietary supplementation with omega-3 in cystic fibrosis (CF) patients. Journal of Cystic Fibrosis 2005;4 Suppl:S88. [CFGD REGISTER: GN109a]
Safai‐Kutti 1991 {published data only}
-
- Safai-Kutti S, Selin E, Larsson S, Jagenburg R, Sten G, Kjellmer I. Zinc therapy in children with cystic fibrosis. Beitraege zur Infusionstherapie 1991;27:104-14. [CFGD REGISTER: GN36] - PubMed
Sagel 2011 {published data only}
Sagel 2018 {published data only}
-
- Jain R, Khan U, Baines A, Sagel SD. Biomarkers of inflammation and oxidative stress in CF: implications for antiinflammatory drug development. Pediatric Pulmonology 2016;51 Suppl 45:262. [CFGD REGISTER: GN265b]
-
- Sagel SD, Baines A, Abdulhamid I, Borowitz D, Clancy JP, Daines C, et al. Effects of an antioxidant-enriched multivitamin supplement on inflammation and oxidative stress in CF. Pediatric Pulmonology 2016;51 Suppl 45:283. [CFGD REGISTER: GN265a]
Tangpricha 2017 {published data only}
-
- NCT01426256. Vitamin D for enhancing the immune system in cystic fibrosis. clinicaltrials.gov/show/NCT01426256 (first received 31 August 2011). [CFGD REGISTER: GN262b]
-
- Tangpricha V, Michalski E, Lukemire J, Binongo J, Judd S, Ziegler T, et al. High-dose vitamin D3 during acute pulmonary exacerbation in cystic fibrosis: a multi-center, double-blind, placebo-controlled trial. Pediatric Pulmonology 2017;52 Suppl 47:471-2. [CFGD REGISTER: GN262e]
-
- Tangpricha V, Smith EM, Binongo J, Judd SE, Ziegler TR, Walker S, et al. The vitamin D for enhancing the immune system in cystic fibrosis (DISC) trial: rationale and design of a multi-center, double-blind, placebo-controlled trial of high dose bolus administration of vitamin D3 during acute pulmonary exacerbation of cystic fibrosis. Contemporary Clinical Trials Communications 2017;6:39-45. [CFGD REGISTER: GN262d] - PMC - PubMed
Winklhofer‐Roob 1995 {published data only}
-
- Winklhofer-Roob BM, Puhl H, Khoschsorur G, van't Hof MA, Esterbauer H, Shmerling DH. Enhanced resistance to oxidation of low density lipoproteins and decreased lipid peroxide formation during beta-carotene supplementation in cystic fibrosis. Free Radical Biology & Medicine 1995;18(5):849-59. - PubMed
-
- Winklhofer-Roob BM, Schlegel-Haueter SE, Khoschsorur G, van't Hof MA, Suter S, Shmerling DH. Neutrophil elastase/alpha 1-proteinase inhibitor complex levels decrease in plasma of cystic fibrosis patients during long-term oral beta-carotene supplementation. Pediatric Research 1996;40(1):130-4. - PubMed
-
- Winklhofer-Roob BM, van't Hof MA, Shmerling DH. Response to oral beta-carotene supplementation in patients with cystic fibrosis: a 16-month follow-up study.[erratum appears in Acta Paediatr 1996 Jan;85(1):124]. Acta Paediatrica 1995 Oct;84(10):1132-6. - PubMed
References to studies awaiting assessment
CARE‐CF‐1 {published data only}
-
- Devereux G, Bourke S, Daines C, Doe S, Dougherty R, Franco R, et al. Care-CF-1 trial: cysteamine, an oral adjunct to standard of care interventions in CF infectious exacerbations. Pediatric Pulmonology 2018;53(S2):260. [CENTRAL: CN-01785552] [CFGD REGISTER: PI309a] [EMBASE: 624049687]
-
- Devereux G, Bourke S, Daines C, Doe S, Dougherty R, Franco R, et al. Evaluating appropriate PROMs in CARE-CF-1 trial: lynovex (cysteamine) an oral adjunct to SOC interventions in cystic fibrosis infectious exacerbations. Journal of Cystic Fibrosis 2019;18(Supplement 1):S23-4. [CENTRAL: CN-02006157] [CFGD REGISTER: PI309b] [EMBASE: 2001976515]
Khorasani 2009 {published data only}
-
- Boskabady M, Nemat Khorasani E, Mansouri F, Soltanian E, Boskabady M. Effect of zinc supplementation on respiratory tract infections in children with cystic fibrosis. Journal of Research in Medical Sciences 2012;17(12):jrms.mui.ac.ir/index.php/jrms/article/view/8833.
-
- Khorasani EN, Mansouri F. Effect of zinc supplementation on respiratory infections in children with cystic fibrosis. In: Proceedings of European Respiratory Society Annual Congress; 2009; Sept 12-16; Vienna, Austria. 2009:722s. [ABSTRACT NO.: P4032] [CENTRAL: 793188] [CFGD REGISTER: GN255]
Puvvadi 2019 {published data only}
-
- Puvvadi R, Mikkelsen H, McCahon L, Ditcham W, Reid D, Lamont I, et al. EDTA combined with nebulised tobramycin improves bacterial clearance and lung function in cystic fibrosis patients with chronic pseudomonas aeruginosa infection. Pediatric Pulmonology 2019;54:299. [CENTRAL: CN-01990631] [CFGD REGISTER: PI319] [EMBASE: 629388437]
Rye 2015 {published data only}
-
- EUCTR2014-002125-35-DE. A phase IIb study of OligoG in subjects with cystic fibrosis colonized with Burkholderia spp. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2014-002125-35-DE (first received 21 July 2014).
-
- NCT02453789. A study of oligog in cystic fibrosis subjects with burkholderia spp. infection. clinicaltrials.gov/show/NCT02453789 (first received 27 May 2015). [CFGD REGISTER: BD223e]
-
- Rye PD, Mahenthiralingam E, Weiser R, Marchesi JR, Opitz P, Trentmann M, et al. Microbiota analysis of samples from a randomized double-blind, placebo-controlled cross-over study of inhaled alginate oligosaccharide (OLIGOG) in cystic fibrosis individuals using aztreonam due to chronic colonization with burkholderia SPP. Pediatric Pulmonology 2015;50 Suppl 41:328. [ABSTRACT NO.: 363] [CENTRAL: 1092173] [CFGD REGISTER: BD223a]
-
- Weiser R, Mahenthiralingham E, Marchesi J, Opitz P, Trentmann M, Rye PD, et al. Standardised cultivation-independent methods to understand the effect of OligoG on Burkholderia and microbial diversity in the CF lung during a clinical trial. Journal of Cystic Fibrosis 2016;15 Suppl 1:S60, Abstract no: 35. [CENTRAL: 1157480] [CFGD REGISTER: BD223b]
-
- Weiser R, Marchesi J, Opitz P, Trentmann M, Rye PD, Onsoyen E, et al. Microbiota analysis of samples from a randomized double-blind, placebo-controlled cross-over study of inhaled alginate oligosaccharide (oligog) in cystic fibrosis individuals using aztreonam due to chronic colonization with burkholderia SPP. Pediatric pulmonology 2016;51:332. [CFGD REGISTER: BD223c]
Walshaw 2014 {published and unpublished data}NCT01465529
-
- NCT01465529. A cross-over study of OligoG in subjects with cystic fibrosis. clinicaltrials.gov/ct2/show/NCT01465529 (first posted 04 November 2011).
-
- Walshaw M, McElvaney G, Williams R, Morice A, Carroll M, Haworth C, et al. A first-in-patient clinical trial demonstrates that inhaled alginate oligosaccharide (OligoG) is well tolerated in cystic fibrosis (CF) patients [abstract]. Journal of Cystic Fibrosis 2014;13 Suppl 2:S58, Abstract no: 45. [CENTRAL: 996544] [CFGD REGISTER: BD202]
Zabner 2009 {unpublished data only}
-
- NCT00928135. Aerosolized hypertonic xylitol versus hypertonic saline in cystic fibrosis (CF) subjects. clinicaltrials.gov/ct2/show/NCT00928135 (first received 25 June 2009).
References to ongoing studies
Pressler 2017 {published data only}
-
- Davies J. Oligo G: a novel mucolytic from basic science to clinical trials. Pediatric Pulmonology 2017;52 Suppl 47:150-1. [CFGD REGISTER: BD235c]
-
- Donaldson SH, Bennett WD, Zeman K, Pressler T, Gow L, Conway J, et al. A first in class mucus conditioning drug-effect of an inhaled alginate oligosaccharide (oligo-g) on mucus clearance in subjects with cystic fibrosis. Pediatric Pulmonology 2017;52 Suppl 47:305. [CFGD REGISTER: BD235d]
-
- Pressler T, Donaldson S, Smerud KT, Myrset AH, OligoG Study Team. A double blind, randomised, placebo-controlled cross over study of inhaled alginate oligosaccharide (OligoG) administered for 28 days in subjects with cystic fibrosis. Journal of Cystic Fibrosis 2017;16 Suppl 1:S1. [CFDG REGISTER: BD235b]
-
- Pressler T, Donaldson SH, Davies JC, Hilde Myrset A. A double-blind, randomised, placebo-controlled cross-over study of inhaled alginate oligosaccharide (OLIGOG) administered for 28 days in subjects with cystic fibrosis. Pediatric Pulmonology 2016;51 Suppl:271. [CFGD REGISTER: BD235a]
-
- Pressler T. A Phase IIb Study of OligoG in Subjects With Cystic Fibrosis (SMR-2984). Clinicaltrials.gov August 2016.
Additional references
Borysowski 2006
-
- Borysowski J, Weber-Dbrowska B, Górski A. Bacteriophage endolysins as a novel class of antibacterial agents. Experimental Biology and Medicine 2006;231(4):366-77. [PMID: ] - PubMed
Dodge 2007
Druesne‐Pecollo 2010
Goss 2007
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Johansson 2008
-
- Johansson EM, Crusz SA, Kolomiets E, Buts L, Kadam RU, Cacciarini M, et al. Inhibition and dispersion of Pseudomonas aeruginosa biofilms by glycopeptide dendrimers targeting the fucose-specific lectin LecB. Chemistry and Biology 2008;15(12):1249-57. [DOI: 10.1016/j.chembiol.2008.10.009] - DOI - PubMed
Langton‐Hewer 2017
Lee 2004
Lo 2018
Quittner 2009
-
- Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire-Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;135(6):1610-8. - PMC - PubMed
Regan 2019
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rosenfeld 2001
Rosenstein 1998
-
- Rosenstein BJ, Cutting GP for the Cystic Fibrosis Foundation consensus panel. The diagnosis of cystic fibrosis: A consensus statement. Journal of Pediatrics 1998;132(4):589-95. [DOI: 10.1016/S0022-3476(98)70344-0 ] - PubMed
UK Cystic Fibrosis Trust 2004
-
- UK Cystic Fibrosis Trust Infection Control Group. Pseudomonas aeruginosa infection in people with cystic fibrosis: Suggestions for prevention and infection control. Report of the UK Cystic Fibrosis Infection Control Group 2004. [ISBN: 0-9540536-9-9]
UK Cystic Fibrosis Trust Antibiotic Working Group
-
- UK Cystic FIbrosis Antibiotic Working Group. Antibiotic Treatment for Cystic Fibrosis - 3rd Edition. Cystic Fibrosis Trust 2009.
References to other published versions of this review
Hurley 2010
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

