A robust multiplex immunofluorescence and digital pathology workflow for the characterisation of the tumour immune microenvironment
- PMID: 32671911
- PMCID: PMC7530793
- DOI: 10.1002/1878-0261.12764
A robust multiplex immunofluorescence and digital pathology workflow for the characterisation of the tumour immune microenvironment
Abstract
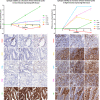
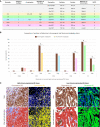
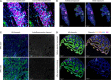
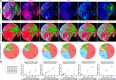
Multiplex immunofluorescence is a powerful tool for the simultaneous detection of tissue-based biomarkers, revolutionising traditional immunohistochemistry. The Opal methodology allows up to eight biomarkers to be measured concomitantly without cross-reactivity, permitting identification of different cell populations within the tumour microenvironment. In this study, we aimed to validate a multiplex immunofluorescence workflow in two complementary multiplex panels and evaluate the tumour immune microenvironment in colorectal cancer (CRC) formalin-fixed paraffin-embedded tissue. We stained CRC and tonsil samples using Opal multiplex immunofluorescence on a Leica BOND RX immunostainer. We then acquired images on an Akoya Vectra Polaris and performed multispectral unmixing using inform. Antibody panels were validated on tissue microarray sections containing cores from six normal tissue types, using qupath for image analysis. Comparisons between chromogenic immunohistochemistry and multiplex immunofluorescence on consecutive sections from the same tissue microarray showed significant correlation (rs > 0.9, P-value < 0.0001), validating both panels. We identified many factors that influenced the quality of the acquired fluorescent images, including biomarker co-expression, staining order, Opal-antibody pairing, sample thickness, multispectral unmixing and biomarker detection order during image analysis. Overall, we report the optimisation and validation of a multiplex immunofluorescence process, from staining to image analysis, ensuring assay robustness. Our multiplex immunofluorescence protocols permit the accurate detection of multiple immune markers in various tissue types, using a workflow that enables rapid processing of samples, above and beyond previous workflows.
Keywords: image analysis; multiplex immunofluorescence; opal methodology.
© 2020 The Authors. Published by FEBS Press and John Wiley & Sons Ltd.
Conflict of interest statement
Prof Phil Quirke has research funding with Roche, GeneFirst and Amgen, previous research funding from Halio, consultancy with Nordlai‐Adlyte and advisory boards with Merck, Amgen and Roche. Prof Manuel Salto‐Tellez is a senior scientific advisor to Philips Computational Pathology and Sonrai Analytics, and has received honoraria from Roche, AstraZeneca, Merck and GSK. These declarations of interest have no relationship with the submitted publication. All other authors declare no competing interests.
Figures



References
-
- Stack EC, Wang C, Roman KA & Hoyt CC (2014) Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of tyramide signal amplification, multispectral imaging and multiplex analysis. Methods 70, 46–58. - PubMed
-
- Tóth ZE & Mezey É (2007) Simultaneous visualization of multiple antigens with tyramide signal amplification using antibodies from the same species. J Histochem Cytochem 55, 545–554. - PubMed
-
- Lim JCT, Yeong JPS, Lim CJ, Ong CCH, Wong SC, Chew VSP, Ahmed SS, Tan PH & Iqbal J (2018) An automated staining protocol for seven‐colour immunofluorescence of human tissue sections for diagnostic and prognostic use. Pathology 50, 333–341. - PubMed

