Tocilizumab for the treatment of adult patients with severe COVID-19 pneumonia: A single-center cohort study
- PMID: 32672860
- PMCID: PMC7404673
- DOI: 10.1002/jmv.26308
Tocilizumab for the treatment of adult patients with severe COVID-19 pneumonia: A single-center cohort study
Abstract
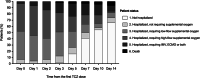
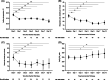
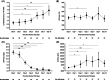
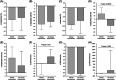
Coronavirus disease 2019 (COVID-19) can lead to a massive cytokine release. The use of the anti-interleukin-6 receptor monoclonal antibody tocilizumab (TCZ) has been proposed in this hyperinflammatory phase, although supporting evidence is limited. We retrospectively analyzed 88 consecutive patients with COVID-19 pneumonia that received at least one dose of intravenous TCZ in our institution between 16 and 27 March 2020. Clinical status from day 0 (first TCZ dose) through day 14 was assessed by a 6-point ordinal scale. The primary outcome was clinical improvement (hospital discharge and/or a decrease of ≥2 points on the 6-point scale) by day 7. Secondary outcomes included clinical improvement by day 14 and dynamics of vital signs and laboratory values. Rates of clinical improvement by days 7 and 14 were 44.3% (39/88) and 73.9% (65/88). Previous or concomitant receipt of subcutaneous interferon-β (adjusted odds ratio [aOR]: 0.23; 95% confidence interval [CI]: 0.06-0.94; P = .041) and serum lactate dehydrogenase more than 450 U/L at day 0 (aOR: 0.25; 95% CI: 0.06-0.99; P = .048) were negatively associated with clinical improvement by day 7. All-cause mortality was 6.8% (6/88). Body temperature and respiratory and cardiac rates significantly decreased by day 1 compared to day 0. Lymphocyte count and pulse oximetry oxygen saturation/FiO2 ratio increased by days 3 and 5, whereas C-reactive protein levels dropped by day 2. There were no TCZ-attributable adverse events. In this observational single-center study, TCZ appeared to be useful and safe as immunomodulatory therapy for severe COVID-19 pneumonia.
Keywords: COVID-19; SARS-CoV-2; immunomodulation; pneumonia; tocilizumab.
© 2020 Wiley Periodicals LLC.
Conflict of interest statement
MFR holds a research contract “Miguel Servet” (CP 18/00073) from the Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation. The other authors declare that there are no conflict of interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

