Pembrolizumab Monotherapy for Recurrent or Metastatic Cutaneous Squamous Cell Carcinoma: A Single-Arm Phase II Trial (KEYNOTE-629)
- PMID: 32673170
- PMCID: PMC7460151
- DOI: 10.1200/JCO.19.03054
Pembrolizumab Monotherapy for Recurrent or Metastatic Cutaneous Squamous Cell Carcinoma: A Single-Arm Phase II Trial (KEYNOTE-629)
Abstract
Purpose: Treatment options are limited for patients with recurrent and/or metastatic (R/M) cutaneous squamous cell carcinoma (cSCC); mortality rates exceed 70% in patients with distant metastases. Here, we present the first interim analysis of the R/M cSCC cohort from the 2-cohort-locally advanced and R/M-phase II KEYNOTE-629 study.
Patients and methods: Patients with R/M cSCC not amenable to surgery or radiation received pembrolizumab 200 mg every 3 weeks. The primary end point was objective response rate per RECIST v1.1. Secondary end points were duration of response, disease control rate, progression-free survival, overall survival, and safety.
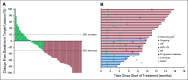
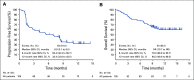
Results: At data cutoff (April 8, 2019), median follow-up of 105 enrolled patients in the R/M cohort was 11.4 months (range, 0.4 to 16.3 months). Objective response rate was 34.3% (95% CI, 25.3% to 44.2%; 4 complete responses, 32 partial responses), and disease control rate was 52.4% (95% CI, 42.4% to 62.2%). Median duration of response was not reached (range, 2.7 to 13.1+ months; '+' refers to ongoing response at data cutoff). Median progression-free survival was 6.9 months (95% CI, 3.1 months to 8.5 months). Median overall survival was not reached (95% CI, 10.7 months to not reached). Treatment-related adverse events occurred in 66.7% of patients (n = 70), the most common of which were pruritus (n = 15; 14.3%), asthenia (n = 14; 13.3%), and fatigue (n = 13; 12.4%). Grade 3 to 5 treatment-related adverse events occurred in 5.7% (n = 6) of patients. One patient died of treatment-related cranial nerve neuropathy.
Conclusion: Pembrolizumab demonstrated effective antitumor activity; clinically meaningful, durable responses; and acceptable safety in primarily elderly patients with R/M cSCC, supporting its use in clinical practice. Pembrolizumab adverse events in this study were consistent with its established safety profile.
Trial registration: ClinicalTrials.gov NCT03284424.
Figures

References
-
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081–1086. - PubMed
-
- Gurney B, Newlands C. Management of regional metastatic disease in head and neck cutaneous malignancy. 1. Cutaneous squamous cell carcinoma. Br J Oral Maxillofac Surg. 2014;52:294–300. - PubMed
-
- Burton KA, Ashack KA, Khachemoune A. Cutaneous squamous cell carcinoma: A review of high-risk and metastatic disease. Am J Clin Dermatol. 2016;17:491–508. - PubMed
-
- Brunner M, Veness MJ, Ch’ng S, et al. Distant metastases from cutaneous squamous cell carcinoma: Analysis of AJCC stage IV. Head Neck. 2013;35:72–75. - PubMed
-
- Veness MJ, Morgan GJ, Palme CE, et al. Surgery and adjuvant radiotherapy in patients with cutaneous head and neck squamous cell carcinoma metastatic to lymph nodes: Combined treatment should be considered best practice. Laryngoscope. 2005;115:870–875. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

