Liver-directed treatments of liver-dominant metastatic leiomyosarcoma
- PMID: 32673206
- PMCID: PMC7490031
- DOI: 10.5152/dir.2020.19405
Liver-directed treatments of liver-dominant metastatic leiomyosarcoma
Abstract
Purpose: The purpose of this study was to determine the safety and efficacy of liver-directed therapies in patients with unresectable metastatic leiomyosarcoma to the liver. Liver-directed therapies included in this study were transarterial chemoembolization with doxorubicin eluting beads (DEB-TACE), yttrium-90 (Y90) radioembolization, and percutaneous microwave ablation.
Methods: This is a single institution retrospective study of unresectable metastatic leiomyosarcoma to the liver treated with DEB-TACE, radioembolization, or microwave ablation. DEB-TACE was performed using 70-150 or 100-300 µ doxorubicin-loaded drug-eluting LC beads. Radioembolization was performed using Y90 glass microspheres. Electronic medical records were retrospectively reviewed to evaluate clinical and biochemical toxicities, tumor response on imaging, overall survival (OS), and liver progression-free survival (PFS).
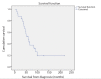
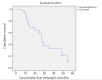
Results: A total of 24 patients with metastatic leiomyosarcoma to the liver who underwent liver-directed treatment were identified (8 males, 16 females; average age, 62.8±11.4 years). Of these patients, 13 underwent DEB-TACE, 6 underwent Y90, and 5 underwent ablation. Three patients received a combination of treatments: one received Y90 followed by DEB-TACE, one received ablation followed by DEB-TACE, and one received ablation followed by Y90. Of the 24 patients, 19 received prior chemotherapy. At 3-month follow-up, grade 1 or 2 lab toxicities were found in 20 patients; 3 patients had grade 3 toxicities. A grade 3 clinical toxicity was reported in one patient. MELD score was 7.5±1.89 at baseline and 8.8±4.2 at 3 months. Median OS was 59 months (95% CI, 39.8-78.2) from diagnosis, 27 months (95% CI, 22.9-31.0) from development of liver metastasis, and 9 months (95% CI, 0-21.4) from first liver-directed treatment. Median liver PFS was 9 months (95% CI, 1.4-16.6).
Conclusion: Treatment with liver-directed therapies for patients with unresectable metastatic leiomyosarcoma to the liver is safe and can improve overall survival, with OS after liver-directed therapy being similar to patients who underwent surgical resection.
Conflict of interest statement
The authors declared no conflicts of interest.
Figures


Comment in
-
Liver-directed Therapies in the Treatment of Leiomyosarcoma Metastases.Radiol Imaging Cancer. 2020 Nov 27;2(6):e209036. doi: 10.1148/rycan.2020209036. eCollection 2020 Nov. Radiol Imaging Cancer. 2020. PMID: 33778754 Free PMC article. No abstract available.
References
-
- Alldinger I, Yang Q, Pilarsky C, Saeger HD, Knoefel WT, Peiper M. Retroperitoneal soft tissue sarcomas: prognosis and treatment of primary and recurrent disease in 117 patients. Anticancer Res. 2006;26:1577–1581. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

