Mutant prion proteins increase calcium permeability of AMPA receptors, exacerbating excitotoxicity
- PMID: 32673372
- PMCID: PMC7365390
- DOI: 10.1371/journal.ppat.1008654
Mutant prion proteins increase calcium permeability of AMPA receptors, exacerbating excitotoxicity
Erratum in
-
Correction: Mutant prion proteins increase calcium permeability of AMPA receptors, exacerbating excitotoxicity.PLoS Pathog. 2020 Dec 22;16(12):e1009174. doi: 10.1371/journal.ppat.1009174. eCollection 2020 Dec. PLoS Pathog. 2020. PMID: 33351850 Free PMC article.
Abstract
Prion protein (PrP) mutations are linked to genetic prion diseases, a class of phenotypically heterogeneous neurodegenerative disorders with invariably fatal outcome. How mutant PrP triggers neurodegeneration is not known. Synaptic dysfunction precedes neuronal loss but it is not clear whether, and through which mechanisms, disruption of synaptic activity ultimately leads to neuronal death. Here we show that mutant PrP impairs the secretory trafficking of AMPA receptors (AMPARs). Specifically, intracellular retention of the GluA2 subunit results in synaptic exposure of GluA2-lacking, calcium-permeable AMPARs, leading to increased calcium permeability and enhanced sensitivity to excitotoxic cell death. Mutant PrPs linked to different genetic prion diseases affect AMPAR trafficking and function in different ways. Our findings identify AMPARs as pathogenic targets in genetic prion diseases, and support the involvement of excitotoxicity in neurodegeneration. They also suggest a mechanistic explanation for how different mutant PrPs may cause distinct disease phenotypes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
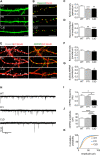





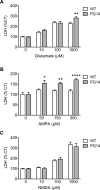

References
-
- Senatore A, Colleoni S, Verderio C, Restelli E, Morini R, Condliffe SB, et al. Mutant PrP suppresses glutamatergic neurotransmission in cerebellar granule neurons by impairing membrane delivery of VGCC alpha(2)delta-1 subunit. Neuron. 2012;74: 300–13. 10.1016/j.neuron.2012.02.027 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

