The Cytokine IL-17A Limits Th17 Pathogenicity via a Negative Feedback Loop Driven by Autocrine Induction of IL-24
- PMID: 32673565
- PMCID: PMC7362799
- DOI: 10.1016/j.immuni.2020.06.022
The Cytokine IL-17A Limits Th17 Pathogenicity via a Negative Feedback Loop Driven by Autocrine Induction of IL-24
Abstract
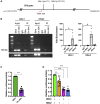
Dysregulated Th17 cell responses underlie multiple inflammatory and autoimmune diseases, including autoimmune uveitis and its animal model, EAU. However, clinical trials targeting IL-17A in uveitis were not successful. Here, we report that Th17 cells were regulated by their own signature cytokine, IL-17A. Loss of IL-17A in autopathogenic Th17 cells did not reduce their pathogenicity and instead elevated their expression of the Th17 cytokines GM-CSF and IL-17F. Mechanistic in vitro studies revealed a Th17 cell-intrinsic autocrine loop triggered by binding of IL-17A to its receptor, leading to activation of the transcription factor NF-κB and induction of IL-24, which repressed the Th17 cytokine program. In vivo, IL-24 treatment ameliorated Th17-induced EAU, whereas silencing of IL-24 in Th17 cells enhanced disease. This regulatory pathway also operated in human Th17 cells. Thus, IL-17A limits pathogenicity of Th17 cells by inducing IL-24. These findings may explain the disappointing therapeutic effect of targeting IL-17A in uveitis.
Keywords: GM-CSF; IL-17; IL-24; Th17; encephalomyelitis; experimental autoimmune uveitis; neuroinflammation; secukinumab.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of Interests U.S. Patent No. 10,512,671, issued December 24, 2019 (R.R.C., W.P.C., R.H., and M.J.M.).
Figures






References
-
- Agarwal R.K., Caspi R.R. Rodent models of experimental autoimmune uveitis. Methods Mol. Med. 2004;102:395–419. - PubMed
-
- Al Omar S., Flanagan B.F., Almehmadi M., Christmas S.E. The effects of IL-17 upon human natural killer cells. Cytokine. 2013;62:123–130. - PubMed
-
- Amadi-Obi A., Yu C.R., Liu X., Mahdi R.M., Clarke G.L., Nussenblatt R.B., Gery I., Lee Y.S., Egwuagu C.E. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat. Med. 2007;13:711–718. - PubMed
-
- Andoh A., Shioya M., Nishida A., Bamba S., Tsujikawa T., Kim-Mitsuyama S., Fujiyama Y. Expression of IL-24, an activator of the JAK1/STAT3/SOCS3 cascade, is enhanced in inflammatory bowel disease. J. Immunol. 2009;183:687–695. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

