Structural insights into DNA loop extrusion by SMC protein complexes
- PMID: 32674008
- PMCID: PMC7612452
- DOI: 10.1016/j.sbi.2020.06.009
Structural insights into DNA loop extrusion by SMC protein complexes
Abstract
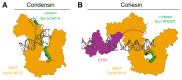
Structural Maintenance of Chromosomes (SMC) protein complexes play key roles in the three-dimensional organization of genomes in all kingdoms of life. Recent insights from chromosome contact mapping experiments and single-molecule imaging assays suggest that these complexes achieve distinct cellular functions by extruding large loops of DNA while they move along the chromatin fiber. In this short review, we summarize recent insights into the molecular architecture of these unconventional DNA motor complexes, their interaction with their DNA substrates, and the remarkable dynamic changes they can undergo during their ATPase reaction cycle.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures



References
-
- Yatskevich S, Rhodes J, Nasmyth K. Organization of Chromosomal DNA by SMC Complexes. Annu Rev Genet. 2019;53:445–482. - PubMed
-
- Nishiyama T. Cohesion and cohesin-dependent chromatin organization. Curr Opin Cell Biol. 2019;58:8–14. - PubMed
-
- Aragon L. The Smc5/6 Complex: New and Old Functions of the Enigmatic Long-Distance Relative. Annu Rev Genet. 2018;52:89–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

