SARS-CoV-2 viral load assessment in respiratory samples
- PMID: 32674034
- PMCID: PMC7235577
- DOI: 10.1016/j.jcv.2020.104439
SARS-CoV-2 viral load assessment in respiratory samples
Abstract
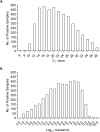
Real-time reverse transcriptase polymerase chain reaction (rRTPCR) has been the main method for diagnosis of SARS-CoV-2 infection in the early stages of the COVID-19 pandemic. De-identified results from upper and lower respiratory samples submitted to a reference laboratory demonstrated a positivity rate of 14.9 % (4428 of 29,713 samples tested). Distribution of results by birth year cohort and specimen type suggested general consistency in mean, median and peak values but higher positivity rates in individuals born from 1964 to 1974. Female patients had a significantly lower positivity rate (P < 0.0001), although similar load mean and median values, compared to males. Overall, 15.3 % (676 of 4428 positive results) of positive results had viral loads greater than 8 log10 copies/mL, with occasional samples exceeding 10 log10 copies/mL. These results support quantitative assessment of SARS-CoV-2 viral load in patient testing and efforts to control viral transmission.
Keywords: RT-PCR; SARS-CoV-2; Viral load diagnostic.
Copyright © 2020. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors have no conflicts of interest to declare.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous