A Viral Toolbox of Genetically Encoded Fluorescent Synaptic Tags
- PMID: 32674057
- PMCID: PMC7363701
- DOI: 10.1016/j.isci.2020.101330
A Viral Toolbox of Genetically Encoded Fluorescent Synaptic Tags
Abstract
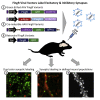
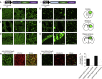
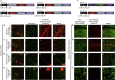
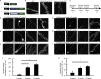
Fibronectin intrabodies generated with mRNA display (FingRs) are a recently developed tool for labeling excitatory or inhibitory synapses, with the benefit of not altering endogenous synaptic protein expression levels or synaptic transmission. Here, we generated a viral vector FingR toolbox that allows for multi-color, neuron-type-specific labeling of excitatory or inhibitory synapses in multiple brain regions. We screened various fluorophores, FingR fusion configurations, and transcriptional control regulations in adeno-associated virus (AAV) and retrovirus vector designs. We report the development of a red FingR variant and demonstrated dual labeling of excitatory and inhibitory synapses in the same cells. Furthermore, we developed cre-inducible FingR AAV variants and demonstrated their utility, finding that the density of inhibitory synapses in aspiny striatal cholinergic interneurons remained unchanged in response to dopamine depletion. Finally, we generated FingR retroviral vectors, which enabled us to track the development of excitatory and inhibitory synapses in hippocampal adult-born granule cells.
Keywords: Biological Sciences; Neuroscience; Techniques in Neuroscience.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing financial interests.
Figures

References
-
- Bindels D.S., Haarbosch L., van Weeren L., Postma M., Wiese K.E., Mastop M., Aumonier S., Gotthard G., Royant A., Hink M.A., Gadella T.W.J., Jr. mScarlet: a bright monomeric red fluorescent protein for cellular imaging. Nat. Methods. 2017;14:1–12. - PubMed
-
- Cearley C.N., Wolfe J.H. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol. Ther. 2006;13:528–537. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

