Direct On-Chip Differentiation of Intestinal Tubules from Induced Pluripotent Stem Cells
- PMID: 32674311
- PMCID: PMC7404294
- DOI: 10.3390/ijms21144964
Direct On-Chip Differentiation of Intestinal Tubules from Induced Pluripotent Stem Cells
Abstract
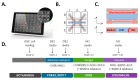
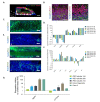
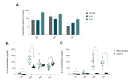
Intestinal organoids have emerged as the new paradigm for modelling the healthy and diseased intestine with patient-relevant properties. In this study, we show directed differentiation of induced pluripotent stem cells towards intestinal-like phenotype within a microfluidic device. iPSCs are cultured against a gel in microfluidic chips of the OrganoPlate, in which they undergo stepwise differentiation. Cells form a tubular structure, lose their stem cell markers and start expressing mature intestinal markers, including markers for Paneth cells, enterocytes and neuroendocrine cells. Tubes develop barrier properties as confirmed by transepithelial electrical resistance (TEER). Lastly, we show that tubules respond to pro-inflammatory cytokine triggers. The whole procedure for differentiation lasts 14 days, making it an efficient process to make patient-specific organoid tubules. We anticipate the usage of the platform for disease modelling and drug candidate screening.
Keywords: 3D cell culture; directed differentiation; gut-on-a-chip; iPSC; intestinal inflammation; intestinal organoids; microfluidics; organ-on-a-chip.
Conflict of interest statement
E.N., K.K., D.K., A.N. and P.V. are employees of Mimetas BV, which is marketing the OrganoPlate and the OrganoTEER. P.V. is a shareholder in Mimetas BV. OrganoPlate is a registered trademark of Mimetas BV. The authors have no additional financial interests.
Figures

Similar articles
-
A xenogeneic-free system generating functional human gut organoids from pluripotent stem cells.JCI Insight. 2017 Jan 12;2(1):e86492. doi: 10.1172/jci.insight.86492. JCI Insight. 2017. PMID: 28097227 Free PMC article.
-
Human pluripotent stem cell-derived intestinal organoids for pharmacokinetic studies.Eur J Cell Biol. 2025 Jun;104(2):151489. doi: 10.1016/j.ejcb.2025.151489. Epub 2025 Apr 1. Eur J Cell Biol. 2025. PMID: 40199084
-
In vitro and in vivo imaging and tracking of intestinal organoids from human induced pluripotent stem cells.FASEB J. 2018 Jan;32(1):111-122. doi: 10.1096/fj.201700504R. Epub 2017 Aug 29. FASEB J. 2018. PMID: 28855280
-
[From human pluripotent stem cells to custom-made intestinal organoids].Med Sci (Paris). 2019 Jun-Jul;35(6-7):549-555. doi: 10.1051/medsci/2019096. Epub 2019 Jul 5. Med Sci (Paris). 2019. PMID: 31274085 Review. French.
-
hPSC-derived organoids: models of human development and disease.J Mol Med (Berl). 2021 Apr;99(4):463-473. doi: 10.1007/s00109-020-01969-w. Epub 2020 Aug 28. J Mol Med (Berl). 2021. PMID: 32857169 Free PMC article. Review.
Cited by
-
Revolutionizing immune research with organoid-based co-culture and chip systems.Clin Exp Immunol. 2024 Sep 16;218(1):40-54. doi: 10.1093/cei/uxae004. Clin Exp Immunol. 2024. PMID: 38280212 Free PMC article. Review.
-
Development of Polymeric Nanoparticles for Blood-Brain Barrier Transfer-Strategies and Challenges.Adv Sci (Weinh). 2021 Mar 7;8(10):2003937. doi: 10.1002/advs.202003937. eCollection 2021 May. Adv Sci (Weinh). 2021. PMID: 34026447 Free PMC article. Review.
-
Organoids: fundamentals, present and future.Rev Peru Med Exp Salud Publica. 2022 Apr-Jun;39(2):227-235. doi: 10.17843/rpmesp.2022.392.10203. Epub 2022 Sep 2. Rev Peru Med Exp Salud Publica. 2022. PMID: 36477325 Free PMC article.
-
Current applications of intestinal organoids: a review.Stem Cell Res Ther. 2024 May 31;15(1):155. doi: 10.1186/s13287-024-03768-3. Stem Cell Res Ther. 2024. PMID: 38816841 Free PMC article. Review.
-
The Growing Importance of Three-Dimensional Models and Microphysiological Systems in the Assessment of Mycotoxin Toxicity.Toxins (Basel). 2023 Jun 29;15(7):422. doi: 10.3390/toxins15070422. Toxins (Basel). 2023. PMID: 37505691 Free PMC article. Review.
References
-
- Mattei G., Giusti S., Ahluwalia A. Design criteria for generating physiologically relevant in vitro models in bioreactors. Processes. 2014;2:548–569. doi: 10.3390/pr2030548. - DOI
-
- Akazawa T., Yoshida S., Ohnishi S., Kanazu T., Kawai M., Takahashi K. Application of intestinal epithelial cells differentiated from human induced pluripotent stem cells for studies of prodrug hydrolysis and drug absorption in the small intestine. Drug Metab. Dispos. 2018;46:1497–1506. doi: 10.1124/dmd.118.083246. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

