Beclin1 Binds to Enterovirus 71 3D Protein to Promote the Virus Replication
- PMID: 32674313
- PMCID: PMC7411969
- DOI: 10.3390/v12070756
Beclin1 Binds to Enterovirus 71 3D Protein to Promote the Virus Replication
Abstract
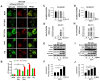
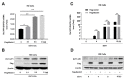
Enterovirus 71 (EV71) is the main pathogen causing hand-foot-mouth disease (HFMD) in infants and children, which can also lead to severe neurological diseases and even death. Therefore, understanding the replication mechanism of EV71 is of great significance for the prevention and control of EV71-induced diseases. Beclin1 (BECN1, a mammalian homologue of ATG6 in yeast) is an important core protein for the initiation and the normal process of autophagy in cells. In addition to its involvement in autophagy, Beclin1 has also been reported to play an important role in cancer and innate immune signaling pathways. However, the role of Beclin1 in EV71 replication remains elusive. Here, we primarily found that Beclin1 facilitates EV71 replication in human rhabdomyosarcoma (RD) cells and the autophagy was actually induced, but Beclin1 was not significantly affected at either mRNA level or protein level during early EV71 infection. Further studies discovered that Beclin1 could interacts with EV71 non-structural protein 3D mainly through its evolutionary conserved domain (ECD) and coiled-coiled domain (CCD), thus promoting the replication of EV71 in human rhabdomyosarcoma (RD) cells and human astroglioma (U251) cells. Collectively, we reveal a novel regulatory mechanism associated with Beclin1 to promote EV71 replication, thus providing a potential therapeutic target for the prevention and control of EV71-associated diseases.
Keywords: Beclin1; EV71; EV71 non-structural protein 3D; autophagy; enterovirus 71.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Enterovirus 71 VP1 Protein Regulates Viral Replication in SH-SY5Y Cells via the mTOR Autophagy Signaling Pathway.Viruses. 2019 Dec 20;12(1):11. doi: 10.3390/v12010011. Viruses. 2019. PMID: 31861844 Free PMC article.
-
SUMO Modification Stabilizes Enterovirus 71 Polymerase 3D To Facilitate Viral Replication.J Virol. 2016 Nov 14;90(23):10472-10485. doi: 10.1128/JVI.01756-16. Print 2016 Dec 1. J Virol. 2016. PMID: 27630238 Free PMC article.
-
Fibronectin Facilitates Enterovirus 71 Infection by Mediating Viral Entry.J Virol. 2018 Apr 13;92(9):e02251-17. doi: 10.1128/JVI.02251-17. Print 2018 May 1. J Virol. 2018. PMID: 29467312 Free PMC article.
-
Recent Progress on Functional Genomics Research of Enterovirus 71.Virol Sin. 2019 Feb;34(1):9-21. doi: 10.1007/s12250-018-0071-9. Epub 2018 Dec 14. Virol Sin. 2019. PMID: 30552635 Free PMC article. Review.
-
[Molecular Mechanism of Action of hnRNP K and RTN3 in the Replication of Enterovirus 71].Bing Du Xue Bao. 2015 Mar;31(2):197-200. Bing Du Xue Bao. 2015. PMID: 26164948 Review. Chinese.
Cited by
-
GRP78 recognizes EV-F 3D protein and activates NF-κB to repress virus replication by interacting with CHUK/IKBKB.J Virol. 2024 Jun 13;98(6):e0026824. doi: 10.1128/jvi.00268-24. Epub 2024 May 22. J Virol. 2024. PMID: 38775480 Free PMC article.
-
TRAF3 activates STING-mediated suppression of EV-A71 and target of viral evasion.Signal Transduct Target Ther. 2023 Feb 24;8(1):79. doi: 10.1038/s41392-022-01287-2. Signal Transduct Target Ther. 2023. PMID: 36823147 Free PMC article.
-
Dysregulated autophagy contributes to the pathogenesis of enterovirus A71 infection.Cell Biosci. 2020 Dec 9;10(1):142. doi: 10.1186/s13578-020-00503-2. Cell Biosci. 2020. PMID: 33298183 Free PMC article. Review.
-
Hijacking the Host Cell for Replication: Pro-Viral Host Factors Involved in EVA71 Infection.Int J Mol Sci. 2025 Aug 19;26(16):7992. doi: 10.3390/ijms26167992. Int J Mol Sci. 2025. PMID: 40869312 Free PMC article. Review.
-
In Memory of the Virologist Jianguo Wu, 1957-2022.Viruses. 2023 Aug 17;15(8):1754. doi: 10.3390/v15081754. Viruses. 2023. PMID: 37632095 Free PMC article.
References
-
- Nathalie J., Schmidt E.H. An Apparently New Enterovirus Isolated from Patients with Disease of the Central Nervous System. J. Infect. Dis. 1974:129. - PubMed
-
- Chang L.Y., Lin T.Y., Hsu K.H., Huang Y.C., Lin K.L., Hsueh C., Shih S.R., Ning H.C., Hwang M.S., Wang H.S., et al. Clinical features and risk factors of pulmonary oedema after enterovirus-71-related hand, foot, and mouth disease. Lancet. 1999;354:1682–1686. doi: 10.1016/S0140-6736(99)04434-7. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

