Molecular Characterization and Determination of Relative Cytokine Expression in Naturally Infected Day-Old Chicks with Chicken Astrovirus Associated to White Chick Syndrome
- PMID: 32674433
- PMCID: PMC7401566
- DOI: 10.3390/ani10071195
Molecular Characterization and Determination of Relative Cytokine Expression in Naturally Infected Day-Old Chicks with Chicken Astrovirus Associated to White Chick Syndrome
Abstract
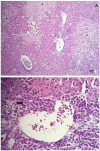
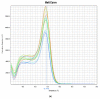
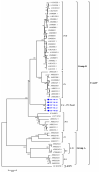
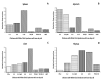
White chick syndrome (WCS) is an emergent disease that affects hatchability and hatched chicks, resulting in high mortality and economic losses, and is related to chicken astrovirus (CAstV). This syndrome has been reported in several countries worldwide, and groups A iii and B vi of CAstV have been determined; however, in Brazil, the virus has not been genotyped. The innate immunity of chicks affected by WCS or any CAstV is poorly understood and studied, and it is important to determine whether relative cytokine expression occurs during the early stages of the life of chicks. The aim of the present investigation is to detect and molecularly characterize CAstV associated with WCS, examine the macroscopic and microscopic lesions in the jejunum and spleen, and determine cytokine expression in the jejunum, liver, spleen and thymus of chicks naturally infected with WCS. To do so, we applied a pathological and molecular approach for CAstV detection and characterization, as well as the quantification of the relative mRNA expression of several cytokine genes. The phylogenetic analyses of the sequences obtained herein classified CAstV as uniquely belonging to group B iv, showing a high similarity of nucleotides (NT) (75.7-80.6%) and amino acids (AA) (84.2-89.9%) with the members of group B and a low similarity of NT (46.7-47.9%) and AA (37.8-38.9%) with the virus belonging in group A. CAstV was also detected and quantified in the serum, spleen, thymus and jejunum, the latter being the organ where CAstV had the highest viral concentration. However, this organ did not present any microscopical alterations. In contrast, we observed necrotic hepatitis in the liver of the affected subjects. On the other hand, we observed the activation of several T helper 1 (Th1)- and T helper 2 (Th2)-cytokines (IFN-γ, IL-2, IL-8, IL-12p40, IL-15, TGF-β4, TNF-SF-15 and t-BET), without being able to control the viral replication due to the high concentration of viral particles in some organs, principally in the gut. One possible role of these cytokines is contributing to the control of inflammation and cell protection of intestinal cells, principally during the early activation of immune responses. However, the fact that these responses are not mature enough to control the viral infection means that more studies need to be carried out to elucidate this topic.
Keywords: Th1; Th2; chicken astrovirus; cytokines; white chicks.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

