Interleaved Array Transducer with Polarization Inversion Technique to Implement Ultrasound Tissue Harmonic Imaging
- PMID: 32674455
- PMCID: PMC7411699
- DOI: 10.3390/s20143915
Interleaved Array Transducer with Polarization Inversion Technique to Implement Ultrasound Tissue Harmonic Imaging
Abstract
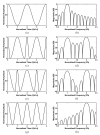
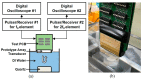
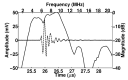
In ultrasound tissue harmonic imaging (THI), it is preferred that the bandwidth of the array transducer covers at least the fundamental frequency f0 for transmission and the second harmonic frequency 2f0 for reception. However, it is challenging to develop an array transducer with a broad bandwidth due to the single resonance characteristics of piezoelectric materials. In this study, we present an improved interleaved array transducer suitable for THI and a dedicated transducer fabrication scheme. The proposed array transducer has a novel structure in which conventional elements exhibiting f0 resonant frequency and polarization-inverted elements exhibiting 2f0 resonant frequency are alternately located, and the thicknesses of all piezoelectric elements are identical. The performance of the proposed method was demonstrated by finite element analysis (FEA) simulations and experiments using a fabricated prototype array transducer. Using the proposed technique, f0 and 2f0 frequency ultrasounds can be efficiently transmitted and received, respectively, resulting in a 90% broad bandwidth feature of the transducer. Thus, the proposed technique can be one of the potential ways to implement high resolution THI.
Keywords: finite element analysis (FEA) simulation; interdigital bonding process; interleaved array transducer; polarization inversion technique; tissue harmonic imaging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Hedrick W.R., Metzger L. Tissue harmonic imaging: A review. J. Diagn. Med. Sonogr. 2005;21:183–189. doi: 10.1177/8756479305276477. - DOI
-
- Averkiou M.A., Roundhill D.N., Powers J.E. A new imaging technique based on the nonlinear properties of tissues; Proceedings of the IEEE Ultrasonic Symposium; Toronto, ON, Canada. 5–8 October 1997; pp. 1561–1566.
MeSH terms
LinkOut - more resources
Full Text Sources

