Multimodality Imaging in the Diagnostic Work-Up of Endocarditis and Cardiac Implantable Electronic Device (CIED) Infection
- PMID: 32674517
- PMCID: PMC7408824
- DOI: 10.3390/jcm9072237
Multimodality Imaging in the Diagnostic Work-Up of Endocarditis and Cardiac Implantable Electronic Device (CIED) Infection
Abstract
Infective endocarditis (IE) is a serious cardiac condition, which includes a wide range of clinical presentations, with varying degrees of severity. The diagnosis is multifactorial and a proper characterization of disease requires the identification of the primary site of infection (usually the cardiac valve) and the search of secondary systemic complications. Early depiction of local complications or distant embolization has a great impact on patient management and prognosis, as it may induce to aggressive antibiotic treatment or, in more advanced cases, cardiac surgery. In this setting, the multimodality imaging has assumed a pivotal role in the clinical decision making and it requires the physician to be aware of the advantages and disadvantages of each imaging technique. Echocardiography is the first imaging test, but it has several limitations. Therefore, the integration with other imaging modalities (computed tomography, magnetic resonance imaging, nuclear imaging) becomes often necessary. Different strategies should be applied depending on whether the infection is suspected or already ascertained, whether located in native or prosthetic valves, in the left or right chambers, or if it involves an implanted cardiac device. In addition, detection of extracardiac IE-related lesions is crucial for a correct management and treatment. The aim of this review is to illustrate strengths and weaknesses of the various methods in the most common clinical scenarios.
Keywords: Infective endocarditis; computed tomography; echocardiography; endocarditis team; magnetic resonance imaging; multimodality imaging; nuclear imaging; positron emission tomography.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
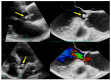
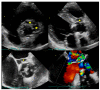
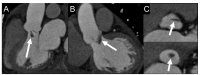
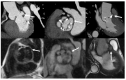



References
-
- Habib G., Lancellotti P., Antunes M.J., Bongiorni M.G., Casalta J.P., Del Zotti F., Dulgheru R., El Khoury G., Erba P.A., Iung B., et al. 2015 ESC guidelines for the management of infective endocarditis: The task force for the management of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM) Eur. Heart J. 2015;36:3075–3128. - PubMed
-
- Habib G., Derumeaux G., Avierinos J.F., Casalta J.P., Jamal F., Volot F., Garcia M., Lefevre J., Biou F., Maximovitch-Rodaminoff A., et al. Value and limitations of the duke criteria for the diagnosis of infective endocarditis. J. Am. Coll. Cardiol. 1999;33:2023–2029. doi: 10.1016/S0735-1097(99)00116-3. - DOI - PubMed
-
- Swart L.E., Scholtens A.M., Tanis W., Nieman K., Bogers A., Verzijlbergen F.J., Krestin G.P., Roos-Hesselink J.W., Budde R.P.J. 18F-fluorodeoxyglucose positron emission/computed tomography and computed tomography angiography in prosthetic heart valve endocarditis: From guidelines to clinical practice. Eur. Heart J. 2018;39:3739–3749. doi: 10.1093/eurheartj/ehx784. - DOI - PubMed

