Human Organs-on-Chips for Virology
- PMID: 32674988
- PMCID: PMC7357975
- DOI: 10.1016/j.tim.2020.06.005
Human Organs-on-Chips for Virology
Abstract
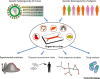
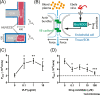
While conventional in vitro culture systems and animal models have been used to study the pathogenesis of viral infections and to facilitate development of vaccines and therapeutics for viral diseases, models that can accurately recapitulate human responses to infection are still lacking. Human organ-on-a-chip (Organ Chip) microfluidic culture devices that recapitulate tissue-tissue interfaces, fluid flows, mechanical cues, and organ-level physiology have been developed to narrow the gap between in vitro experimental models and human pathophysiology. Here, we describe how recent developments in Organ Chips have enabled re-creation of complex pathophysiological features of human viral infections in vitro.
Keywords: microfluidics; microphysiological system; organ-on-a-chip; viral infections; virus.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Microfluidic Organs-on-a-Chip for Modeling Human Infectious Diseases.Acc Chem Res. 2021 Sep 21;54(18):3550-3562. doi: 10.1021/acs.accounts.1c00411. Epub 2021 Aug 29. Acc Chem Res. 2021. PMID: 34459199
-
Human Lung Small Airway-on-a-Chip Protocol.Methods Mol Biol. 2017;1612:345-365. doi: 10.1007/978-1-4939-7021-6_25. Methods Mol Biol. 2017. PMID: 28634955
-
Developmentally inspired human 'organs on chips'.Development. 2018 May 18;145(16):dev156125. doi: 10.1242/dev.156125. Development. 2018. PMID: 29776965 Free PMC article.
-
Modelling cancer in microfluidic human organs-on-chips.Nat Rev Cancer. 2019 Feb;19(2):65-81. doi: 10.1038/s41568-018-0104-6. Nat Rev Cancer. 2019. PMID: 30647431 Review.
-
Modeling the Human Body on Microfluidic Chips.Trends Biotechnol. 2021 Aug;39(8):838-852. doi: 10.1016/j.tibtech.2021.01.004. Epub 2021 Feb 10. Trends Biotechnol. 2021. PMID: 33581889 Review.
Cited by
-
Molecular Communications in Viral Infections Research: Modeling, Experimental Data, and Future Directions.IEEE Trans Mol Biol Multiscale Commun. 2021 Apr 15;7(3):121-141. doi: 10.1109/TMBMC.2021.3071780. eCollection 2021 Sep. IEEE Trans Mol Biol Multiscale Commun. 2021. PMID: 35782714 Free PMC article.
-
Fluorescent toys 'n' tools lighting the way in fungal research.FEMS Microbiol Rev. 2021 Sep 8;45(5):fuab013. doi: 10.1093/femsre/fuab013. FEMS Microbiol Rev. 2021. PMID: 33595628 Free PMC article.
-
Vasculature-on-a-chip technologies as platforms for advanced studies of bacterial infections.Biomicrofluidics. 2024 Mar 25;18(2):021503. doi: 10.1063/5.0179281. eCollection 2024 Mar. Biomicrofluidics. 2024. PMID: 38560344 Free PMC article. Review.
-
Ex vivo study of neuroinvasive and neurotropic viruses: what is current and what is next.FEMS Microbiol Rev. 2025 Jan 14;49:fuaf024. doi: 10.1093/femsre/fuaf024. FEMS Microbiol Rev. 2025. PMID: 40498321 Free PMC article. Review.
-
Modeling SARS-CoV-2 and influenza infections and antiviral treatments in human lung epithelial tissue equivalents.Commun Biol. 2022 Aug 12;5(1):810. doi: 10.1038/s42003-022-03753-7. Commun Biol. 2022. PMID: 35962146 Free PMC article.
References
-
- Bhatia S.N., Ingber D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014;32:760–772. - PubMed
-
- Sontheimer-Phelps A., et al. Modelling cancer in microfluidic human organs-on-chips. Nat. Rev. Cancer. 2019;19:65–81. - PubMed
-
- Rosellini A., et al. Enhanced in vitro virus expression using 3-dimensional cell culture spheroids for infection. J. Virol. Methods. 2019;265:99–104. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

