Mitochondrial dynamics during spermatogenesis
- PMID: 32675215
- PMCID: PMC7375475
- DOI: 10.1242/jcs.235937
Mitochondrial dynamics during spermatogenesis
Abstract
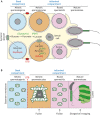
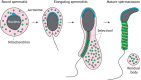
Mitochondrial fusion and fission (mitochondrial dynamics) are homeostatic processes that safeguard normal cellular function. This relationship is especially strong in tissues with constitutively high energy demands, such as brain, heart and skeletal muscle. Less is known about the role of mitochondrial dynamics in developmental systems that involve changes in metabolic function. One such system is spermatogenesis. The first mitochondrial dynamics gene, Fuzzy onions (Fzo), was discovered in 1997 to mediate mitochondrial fusion during Drosophila spermatogenesis. In mammals, however, the role of mitochondrial fusion during spermatogenesis remained unknown for nearly two decades after discovery of Fzo Mammalian spermatogenesis is one of the most complex and lengthy differentiation processes in biology, transforming spermatogonial stem cells into highly specialized sperm cells over a 5-week period. This elaborate differentiation process requires several developmentally regulated mitochondrial and metabolic transitions, making it an attractive model system for studying mitochondrial dynamics in vivo We review the emerging role of mitochondrial biology, and especially its dynamics, during the development of the male germ line.
Keywords: Membrane fission; Membrane fusion; Mitochondrial dynamics; Spermatogenesis.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures



References
-
- Baklouti-Gargouri S., Ghorbel M., Ben Mahmoud A., Mkaouar-Rebai E., Cherif M., Chakroun N., Sellami A., Fakhfakh F. and Ammar-Keskes L. (2014). Identification of a novel m.9588G>a missense mutation in the mitochondrial COIII gene in asthenozoospermic Tunisian infertile men. J. Assist. Reprod. Genet. 31, 595-600. 10.1007/s10815-014-0187-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

