Huntington's disease alters human neurodevelopment
- PMID: 32675289
- PMCID: PMC7859879
- DOI: 10.1126/science.aax3338
Huntington's disease alters human neurodevelopment
Abstract
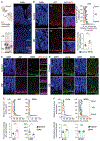
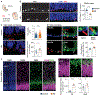
Although Huntington's disease is a late-manifesting neurodegenerative disorder, both mouse studies and neuroimaging studies of presymptomatic mutation carriers suggest that Huntington's disease might affect neurodevelopment. To determine whether this is actually the case, we examined tissue from human fetuses (13 weeks gestation) that carried the Huntington's disease mutation. These tissues showed clear abnormalities in the developing cortex, including mislocalization of mutant huntingtin and junctional complex proteins, defects in neuroprogenitor cell polarity and differentiation, abnormal ciliogenesis, and changes in mitosis and cell cycle progression. We observed the same phenomena in Huntington's disease mouse embryos, where we linked these abnormalities to defects in interkinetic nuclear migration of progenitor cells. Huntington's disease thus has a neurodevelopmental component and is not solely a degenerative disease.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures


Comment in
-
Huntington disease alters early neurodevelopment.Nat Rev Neurol. 2020 Sep;16(9):459. doi: 10.1038/s41582-020-0399-2. Nat Rev Neurol. 2020. PMID: 32759998 No abstract available.
-
An early start to Huntington's disease.Science. 2020 Aug 14;369(6505):771-772. doi: 10.1126/science.abd6215. Science. 2020. PMID: 32792383 No abstract available.
-
Developmental disruptions.Nat Rev Neurosci. 2020 Nov;21(11):592-593. doi: 10.1038/s41583-020-0377-0. Nat Rev Neurosci. 2020. PMID: 32873935 No abstract available.
-
Huntington's Disease: Les Jeux Sont Faits?Trends Mol Med. 2020 Oct;26(10):889-890. doi: 10.1016/j.molmed.2020.08.004. Epub 2020 Sep 4. Trends Mol Med. 2020. PMID: 32893090
-
The Huntington's mutation implicated in early development.Nat Med. 2020 Sep;26(9):1331. doi: 10.1038/s41591-020-1068-0. Nat Med. 2020. PMID: 32908281 No abstract available.
References
-
- Duyao MP et al., Science 269, 407–410 (1995). - PubMed
-
- Zeitlin S, Liu JP, Chapman DL, Papaioannou VE, Efstratiadis A, Nat. Genet 11, 155–163 (1995). - PubMed
-
- Reiner A, Dragatsis I, Zeitlin S, Goldowitz D, Mol. Neurobiol 28, 259–276 (2003). - PubMed
-
- Godin JD et al., Neuron 67, 392–406 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

