This is a preprint.
SARS-CoV-2 Seroprevalence Among Parturient Women
- PMID: 32676623
- PMCID: PMC7359548
- DOI: 10.1101/2020.07.08.20149179
SARS-CoV-2 Seroprevalence Among Parturient Women
Update in
-
SARS-CoV-2 seroprevalence among parturient women in Philadelphia.Sci Immunol. 2020 Jul 29;5(49):eabd5709. doi: 10.1126/sciimmunol.abd5709. Sci Immunol. 2020. PMID: 32727884 Free PMC article.
Abstract
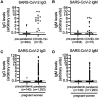
Limited data are available for pregnant women affected by SARS-CoV-2. Serological tests are critically important to determine exposure and immunity to SARS-CoV-2 within both individuals and populations. We completed SARS-CoV-2 serological testing of 1,293 parturient women at two centers in Philadelphia from April 4 to June 3, 2020. We tested 834 pre-pandemic samples collected in 2019 and 15 samples from COVID-19 recovered donors to validate our assay, which has a ~1% false positive rate. We found 80/1,293 (6.2%) of parturient women possessed IgG and/or IgM SARS-CoV-2-specific antibodies. We found race/ethnicity differences in seroprevalence rates, with higher rates in Black/non-Hispanic and Hispanic/Latino women. Of the 72 seropositive women who also received nasopharyngeal polymerase chain reaction testing during pregnancy, 46 (64%) were positive. Continued serologic surveillance among pregnant women may inform perinatal clinical practices and can potentially be used to estimate seroprevalence within the community.
Figures
References
-
- Abbasi J. The Promise and Peril of Antibody Testing for COVID-19. JAMA [Internet]. 2020. April 17 [cited 2020 Apr 24]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/32301958 - PubMed
-
- Crook D. Evaluation of antibody testing for SARS-CoV-2 using ELISA and lateral flow immunoassays. [cited 2020 May 1]; Available from: 10.1101/2020.04.15.20066407 - DOI
-
- Whitman JD, Hiatt J, Mowery CT, Shy BR, Yu R, Yamamoto TN, et al. Test performance evaluation of SARS-CoV-2 serological assays. medRxiv. 2020. April 29;2020.04.25.20074856.
-
- Vital Statistics Annual Report [Internet]. [cited 2020 Apr 29]. Available from: https://www.health.pa.gov/topics/HealthStatistics/VitalStatistics/PAVita...
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
