Role of the MUC1-C oncoprotein in the acquisition of cisplatin resistance by urothelial carcinoma
- PMID: 32677159
- PMCID: PMC7541007
- DOI: 10.1111/cas.14574
Role of the MUC1-C oncoprotein in the acquisition of cisplatin resistance by urothelial carcinoma
Retraction in
-
RETRACTION: Role of the MUC1-C Oncoprotein in the Acquisition of Cisplatin Resistance by Urothelial Carcinoma.Cancer Sci. 2025 Apr;116(4):1154. doi: 10.1111/cas.70016. Epub 2025 Feb 11. Cancer Sci. 2025. PMID: 39932034 Free PMC article.
Abstract
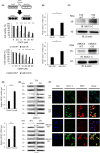
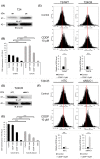
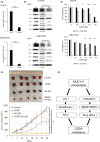
Mucin 1 C-terminal subunit (MUC1-C) has been introduced as a key regulator for acquiring drug resistance in various cancers, but the functional role of MUC1-C in urothelial carcinoma (UC) cells remains unknown. We aimed to elucidate the molecular mechanisms underlying the acquisition of cisplatin (CDDP) resistance through MUC1-C oncoprotein in UC cells. MUC1-C expression was examined immunohistochemically in tumor specimens of 159 UC patients who received CDDP-based perioperative chemotherapy. As a result, moderate to high MUC1-C expression was independently associated with poor survival in UC patients. Using human bladder cancer cell lines and CDDP-resistant (CR) cell lines, we compared the expression levels of MUC1-C, multiple drug resistance 1 (MDR1), the PI3K-AKT-mTOR pathway, and x-cystine/glutamate transporter (xCT) to elucidate the biological mechanisms contributing to the acquisition of chemoresistance. MUC1-C was strongly expressed in CR cell lines, followed with MDR1 expression via activation of the PI3K-AKT-mTOR pathway. MUC1-C also stabilized the expression of xCT, which enhanced antioxidant defenses by increasing intracellular glutathione (GSH) levels. MUC1 down-regulation showed MDR1 inhibition along with PI3K-AKT-mTOR pathway suppression. Moreover, it inhibited xCT stabilization and resulted in significant decreases in intracellular GSH levels and increased reactive oxygen species (ROS) generation. The MUC1-C inhibitor restored sensitivity to CDDP in CR cells and UC murine xenograft models. In conclusion, we found that MUC1-C plays a pivotal role in the acquisition of CDDP resistance in UC cells, and therefore the combined treatment of CDDP with a MUC1-C inhibitor may become a novel therapeutic option in CR UC patients.
Keywords: GO-203; MDR1; MUC1-C; PI3K-AKT-mTOR; urothelial carcinoma; xCT.
© 2020 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
Dr. Donald Kufe holds equity in Genus Oncology and is a consultant to the company. The other authors disclosed no potential conflicts of interest.
Figures


References
-
- Alfred Witjes J, Lebret T, Comperat EM, et al. Updated 2016 EAU guidelines on muscle‐invasive and metastatic bladder cancer. Eur Urol. 2017;71:462‐475. - PubMed
-
- Sternberg CN, Yagoda A, Scher HI, et al. M‐VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for advanced transitional cell carcinoma of the urothelium. J Urol. 1988;139:461‐469. - PubMed
-
- von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068‐3077. - PubMed
-
- Silva R, Vilas‐Boas V, Carmo H, et al. Modulation of P‐glycoprotein efflux pump: induction and activation as a therapeutic strategy. Pharmacol Ther. 2015;149:1‐123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

