Stereoinduction in Metallaphotoredox Catalysis
- PMID: 32677341
- PMCID: PMC8066398
- DOI: 10.1002/anie.202007668
Stereoinduction in Metallaphotoredox Catalysis
Abstract
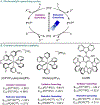
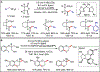
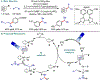
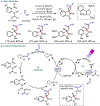
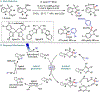
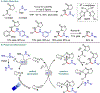
Metallaphotoredox catalysis has evolved into an enabling platform to construct C(sp3 )-hybridized centers under remarkably mild reaction conditions. The cultivation of abundant radical precursor feedstocks has significantly increased the scope of transition-metal-catalyzed cross-couplings, especially with respect to C(sp2 )-C(sp3 ) linkages. In recent years, considerable effort has been devoted to understanding the origin of stereoinduction in dual catalytic processes. In this context, Ni- and Cu-catalyzed transformations have played a predominant role exploiting this mode of catalysis. Herein, we provide a critical overview on recent progress in enantioselective bond formations enabled by Ni- and Cu-catalyzed manifolds. Furthermore, selected stereochemical control elements within the realm of diastereoselective transformations are discussed.
Keywords: cross-coupling; energy transfer; photocatalysis; radical precursors; stereoinduction.
© 2020 Wiley-VCH GmbH.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest.
Figures






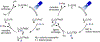
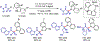

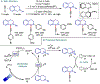

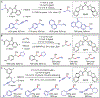

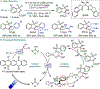
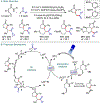

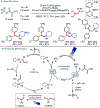
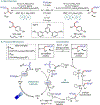
References
-
- Tellis JC, Primer DN, Molander GA, Science 2014, 345, 433–436; - PMC - PubMed
- Zuo Z, Ahneman DT, Chu L, Terrett JA, Doyle AG, MacMillan DW, Science 2014, 345, 437–440; - PMC - PubMed
- Primer DN, Karakaya I, Tellis JC, Molander GA, J. Am. Chem. Soc 2015, 137, 2195–2198; - PMC - PubMed
- Shields BJ, Doyle AG, J. Am. Chem. Soc 2016, 138, 12719–12722. - PMC - PubMed
- For selected reviews, please see: Tellis JC, Kelly CB, Primer DN, Jouffroy M, Patel NR, Molander GA, Acc. Chem. Res 2016, 49, 1429–1439; - PMC - PubMed
- Skubi KL, Blum TR, Yoon TP, Chem. Rev 2016, 116, 10035–10074; - PMC - PubMed
- Milligan JA, Phelan JP, Badir SO, Molander GA, Angew. Chem. Int. Ed 2019, 58, 6152–6163; Angew. Chem. 2019, 131, 6212–6224; - PMC - PubMed
- Twilton J, Le C, Zhang P, Shaw MH, Evans RW, MacMillan DWC, Nat. Rev. Chem 2017, 1, 0052;
- Matsui JK, Lang SB, Heitz DR, Molander GA, ACS Catal 2017, 7, 2563–2575; - PMC - PubMed
- Parasram M, Gevorgyan V, Chem. Soc. Rev 2017, 46, 6227–6240. - PMC - PubMed
-
- Lovering F, Bikker J, Humblet C, J. Med. Chem 2009, 52, 6752–6756; - PubMed
- Lovering F, MedChemComm 2013, 4, 515–519.
-
- For selected examples of complementary radical-based asymmetric Ni- and Cu-catalyzed transformations, please see: Zhao Y, Weix DJ, J. Am. Chem. Soc 2015, 137, 3237–3240; - PMC - PubMed
- Gu Q-S, Li Z-L, Liu X-Y, Acc. Chem. Res 2020, 53, 170–181; - PubMed
- Li Z-L, Fang G-C, Gu Q-S, Liu X-Y, Chem. Soc. Rev 2020, 49, 32–48; - PubMed
- Poremba KE, Dibrell SE, Reisman SE, ACS Catal 2020, 10, 8237–8246. - PMC - PubMed
- For recent reviews on enantioselective reactions in photoinduced systems, please see: Busch J, Knoll DM, Zippel C, Bräse S, Bizzarri C, Dalton Trans 2019, 48, 15338–15357; - PubMed
- Zhang H-H, Chen H, Zhu C, Yu S, Sci. China Chem 2020, 63, 637–647.
-
- Hartwig JF, Organotransition Metal Chemistry: from Bonding to Catalysis, University Science Books, Sausalito, Calif., 2010.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

