Metabolic profiling shows pre-existing mitochondrial dysfunction contributes to muscle loss in a model of ICU-acquired weakness
- PMID: 32677363
- PMCID: PMC7567140
- DOI: 10.1002/jcsm.12597
Metabolic profiling shows pre-existing mitochondrial dysfunction contributes to muscle loss in a model of ICU-acquired weakness
Abstract
Background: Surgery can lead to significant muscle loss, which increases recovery time and associates with increased mortality. Muscle loss is not uniform, with some patients losing significant muscle mass and others losing relatively little, and is likely to be accompanied by marked changes in circulating metabolites and proteins. Determining these changes may help understand the variability and identify novel therapeutic approaches or markers of muscle wasting.
Methods: To determine the association between muscle loss and circulating metabolites, we studied 20 male patients (median age, 70.5, interquartile range, 62.5-75) undergoing aortic surgery. Muscle mass was determined before and 7 days after surgery and blood samples were taken before surgery, and 1, 3, and 7 days after surgery. The circulating metabolome and proteome were determined using commercial services (Metabolon and SomaLogic).
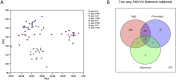
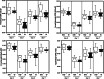
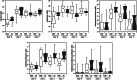
Results: Ten patients lost more than 10% of the cross-sectional area of the rectus femoris (RFCSA ) and were defined as wasting. Metabolomic analysis showed that 557 circulating metabolites were altered following surgery (q < 0.05) in the whole cohort and 104 differed between wasting and non-wasting patients (q < 0.05). Weighted genome co-expression network analysis, identified clusters of metabolites, both before and after surgery, that associated with muscle mass and function (r = -0.72, p = 6 × 10-4 with RFCSA on Day 0, P = 3 × 10-4 with RFCSA on Day 7 and r = -0.73, P = 5 × 10-4 with hand-grip strength on Day 7). These clusters were mainly composed of acyl carnitines and dicarboxylates indicating that pre-existing mitochondrial dysfunction contributes to muscle loss following surgery. Surgery elevated cortisol to the same extent in wasting and non-wasting patients, but the cortisol:cortisone ratio was higher in the wasting patients (Day 3 P = 0.043 and Day 7 P = 0.016). Wasting patients also showed a greater increase in circulating nucleotides 3 days after surgery. Comparison of the metabolome with inflammatory markers identified by SOMAscan® showed that pre-surgical mitochondrial dysfunction was associated with growth differentiation factor 15 (GDF-15) (r = 0.79, P = 2 × 10-4 ) and that GDF-15, interleukin (IL)-8), C-C motif chemokine 23 (CCL-23), and IL-15 receptor subunit alpha (IL-15RA) contributed to metabolic changes in response to surgery.
Conclusions: We show that pre-existing mitochondrial dysfunction and reduced cortisol inactivation contribute to muscle loss following surgery. The data also implicate GDF-15 and IL-15RA in mitochondrial dysfunction.
Keywords: Aortic surgery; Cortisol; Metabolomics; Mitochondrial dysfunction; Muscle wasting.
© 2020 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
P.K. reports personal fees from GSK, outside the submitted work. M.G. reports grants, personal fees, and non‐financial support from GSK, personal fees from BI, personal fees from Silence therapeutics, personal fees from Cell catapult, outside the submitted work; all other authors have no conflicts of interest.
Figures

References
-
- Gore DC, Jahoor F. Deficiency in peripheral glutamine production in pediatric patients with burns. J Burn Care Rehabil. 2000;21:171, discussion 2‐7. - PubMed
-
- Lightfoot A, McArdle A, Griffiths RD. Muscle in defense. Crit Care Med. 2009;37:S384–S390. - PubMed
-
- Biolo G, Fleming RY, Maggi SP, Nguyen TT, Herndon DN, Wolfe RR. Inhibition of muscle glutamine formation in hypercatabolic patients. Clin Sci (Lond). 2000;99:189–194. - PubMed
-
- Kemp PR, Griffiths M, Polkey MI. Muscle wasting in the presence of disease, why is it so variable? Biol Rev Camb Philos Soc. 2019;94:1038–1055. - PubMed
-
- Anker SD, Sharma R. The syndrome of cardiac cachexia. Int J Cardiol. 2002;85:51–66. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

