Autophagy core protein ATG5 is required for elongating spermatid development, sperm individualization and normal fertility in male mice
- PMID: 32677505
- PMCID: PMC8354593
- DOI: 10.1080/15548627.2020.1783822
Autophagy core protein ATG5 is required for elongating spermatid development, sperm individualization and normal fertility in male mice
Abstract
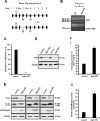
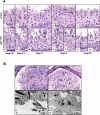
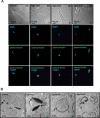
Spermiogenesis is the longest phase of spermatogenesis, with dramatic morphological changes and a final step of spermiation, which involves protein degradation and the removal of excess cytoplasm; therefore, we hypothesized that macroautophagy/autophagy might be involved in the process. To test this hypothesis, we examined the function of ATG5, a core autophagy protein in male germ cell development. Floxed Atg5 and Stra8- iCre mice were crossed to conditionally inactivate Atg5 in male germ cells. In Atg5flox/flox; Stra8- iCre mutant mice, testicular expression of the autophagosome marker LC3A/B-II was significantly reduced, and expression of autophagy receptor SQSTM1/p62 was significantly increased, indicating a decrease in testicular autophagy activity. The fertility of mutant mice was dramatically reduced with about 70% being infertile. Sperm counts and motility were also significantly reduced compared to controls. Histological examination of the mutant testes revealed numerous, large residual bodies in the lumen of stages after their normal resorption within the seminiferous epithelium. The cauda epididymal lumen was filled with sloughed germ cells, large cytoplasmic bodies, and spermatozoa with disorganized heads and tails. Examination of cauda epididymal sperm by electron microscopy revealed misshapen sperm heads, a discontinuous accessory structure in the mid-piece and abnormal acrosome formation and loss of sperm individualization. Immunofluorescence staining of epididymal sperm showed abnormal mitochondria and acrosome distribution in the mutant mice. ATG5 was shown to induce autophagy by mediating multiple signals to maintain normal developmental processes. Our study demonstrated ATG5 is essential for male fertility and is involved in various aspects of spermiogenesis.Abbreviations: AKAP4: a-kinase anchoring protein 4; ATG5: autophagy-related 5; ATG7: autophagy-related 7; ATG10: autophagy-related 10; ATG12: autophagy-related 12; cKO: conditional knockout; DDX4: DEAD-box helicase 4; MAP1LC3/LC3/tg8: microtubule-associated protein 1 light chain 3; PBS: phosphate-buffered saline; PIWIL2/MILI: piwi like RNA-mediated gene silencing 2; RT-PCR: reverse transcription-polymerase chain reaction; SQSTM1/p62: sequestosome 1; TBC: tubulobulbar complexes; WT: wild type.
Keywords: ATG5; Acrosome; autophagy; individualization; male germ cells; male reproduction; mitochondria; spermiogenesis.
Conflict of interest statement
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
