Avoidance and Period-Shortening of Neoadjuvant Chemotherapy Against Triple-Negative Breast Cancer in Stages I and II: Importance of Ki-67 Labeling Index and the Recognition of Apocrine-Type Lesions
- PMID: 32677589
- PMCID: PMC7370551
- DOI: 10.1177/1533033820943246
Avoidance and Period-Shortening of Neoadjuvant Chemotherapy Against Triple-Negative Breast Cancer in Stages I and II: Importance of Ki-67 Labeling Index and the Recognition of Apocrine-Type Lesions
Abstract
Background: Triple-negative breast cancer encompasses heterogeneous subtypes. Neoadjuvant chemotherapy is ineffective against some triple-negative breast cancers, while others show a favorable prognosis despite chemoresistance.
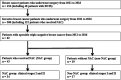
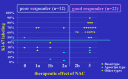
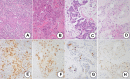
Methods: A total of 51 cases with stages I and II triple-negative breast cancer were analyzed; 34 triple-negative breast cancers treated with neoadjuvant chemotherapy were divided into "good responders" (n = 22), showing therapeutic effect G2b or G3 in surgical specimens, and "poor responders" with therapeutic effect G0, G1a, G1b, and G2a (n = 12). Neoadjuvant chemotherapy was spared in 17 cases (non-neoadjuvant chemotherapy group). Apocrine-type triple-negative breast cancer was defined as triple-negative breast cancer immunoreactive for both androgen receptor and forkhead-box protein A1. Triple-negative breast cancer other than apocrine-type (n = 16) and special types (myoepithelial, medullary, adenoid cystic, and spindle cell carcinomas, n = 6) was categorized as basal-like subtype (n = 29). Prognosis was evaluated in each category.
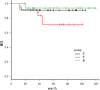
Results: Neoadjuvant chemotherapy provoked significant effects against basal-like triple-negative breast cancer with high Ki-67 labeling (≧50%), and tumor-infiltrating lymphocytes predicted high chemosensitivity. Neoadjuvant chemotherapy was avoidable in triple-negative breast cancer of apocrine- and special types showing low (<50%) Ki-67 labeling. Ten (59%) lesions in the non-neoadjuvant chemotherapy group belonged to the apocrine-type. When clinical complete remission shown by contrast-enhanced magnetic resonance imaging was reached in the course of neoadjuvant chemotherapy against basal-like triple-negative breast cancer, the neoadjuvant chemotherapy period was shortened in 14 (64%) of 22 good responders. Disease-free and overall survival rates were excellent in all groups.
Conclusions: The following 2 hypothetical proposals should be proven by large-scale clinical trials. Immunohistochemical recognition of apocrine-type triple-negative breast cancer with low Ki-67 labeling is important for avoiding ineffective/unnecessary neoadjuvant chemotherapy. By employing appropriate clinical imaging, period-shortening is achievable in basal-like triple-negative breast cancer with high Ki-67 labeling.
Keywords: FOXA1; androgen receptor; apocrine-type; avoidance of chemotherapy; basal-like subtype; period-shortening of chemotherapy; triple-negative breast cancer.
Conflict of interest statement
Figures


References
-
- Ferlay J, Soerjomataram I, Diksit R, et al. Cancer incidence and mortality worldwide: sources, methods and major pattern in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. - PubMed
-
- Center for Cancer Control Information Services, National Cancer Center. Cancer statistics in Japan. 2019. Accessed August 13, 2019 https://ganjoho.jp/en/index.html.
-
- Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000; 406(6797):747–752. - PubMed
-
- Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10(16):5367–5374. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

