Transcriptomic and proteomic analyses of ovarian follicles reveal the role of VLDLR in chicken follicle selection
- PMID: 32677893
- PMCID: PMC7367319
- DOI: 10.1186/s12864-020-06855-w
Transcriptomic and proteomic analyses of ovarian follicles reveal the role of VLDLR in chicken follicle selection
Abstract
Background: Follicle selection in chickens refers to the process of selecting one follicle from a group of small yellow follicles (SY, 6-8 mm in diameter) for development into 12-15 mm hierarchical follicles (usually F6 follicles), which is an important process affecting laying performance in the poultry industry. Although transcriptomic analysis of chicken ovarian follicles has been reported, integrated analysis of chicken follicles for selection by using both transcriptomic and proteomic approaches is still rarely performed. In this study, we compared the proteomes and transcriptomes of SY and F6 follicles in laying hens and identified several genes involved in chicken follicle selection.
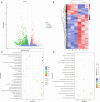
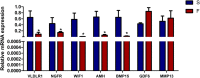
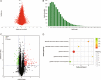
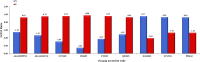
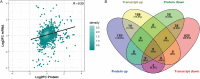
Results: Transcriptomic analysis revealed 855 differentially expressed genes (DEGs) between SY follicles and F6 follicles in laying hens, among which 202 were upregulated and 653 were downregulated. Proteomic analysis revealed 259 differentially expressed proteins (DEPs), including 175 upregulated and 84 downregulated proteins. Among the identified DEGs and DEPs, changes in the expression of seven genes, including VLDLR1, WIF1, NGFR, AMH, BMP15, GDF6 and MMP13, and nine proteins, including VLDLR, VTG1, VTG3, PSCA, APOB, APOV1, F10, ZP2 and ZP3L2, were validated. Further analysis indicated that the mRNA level of chicken VLDLR was higher in F6 follicles than in SY follicles and was also higher in granulosa cells (GCs) than in thecal cells (TCs), and it was stimulated by FSH in GCs.
Conclusions: By comparing the proteomes and transcriptomes of SY and F6 follicles in laying hens, we identified several differentially expressed proteins/genes that might play certain roles in chicken follicle selection. These data may contribute to the identification of functional genes and proteins involved in chicken follicle selection.
Keywords: Chicken; Differentially expressed genes; Differentially expressed proteins; Follicle; Proteome; Transcriptome.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Johnson AL. Ovarian follicle selection and granulosa cell differentiation. Poult Sci. 2014;94(4):781–785. - PubMed
-
- Onagbesan O, Bruggeman V, Decuypere E. Intra-ovarian growth factors regulating ovarian function in avian species: a review. Anim Reprod Sci. 2009;111:121–140. - PubMed
-
- Tilly JL, Kowalski KI, Johnson AL. Stage of ovarian follicular development associated with the initiation of steroidogenic competence in avian granulosa cells. Biol Reprod. 1991;44(2):305–314. - PubMed
-
- Johnson AL, Woods DC. Ovarian dynamics and follicle development. In: Jamieson BGM, editor. Reproductive biology and phylogeny of birds. Enfield (NH): Science Publishers, an imprint of Edenbridge Ltd. 2007; pp: 243–277 (Chapter 6).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

