Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer's disease
- PMID: 32677986
- PMCID: PMC7364557
- DOI: 10.1186/s13024-020-00391-7
Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer's disease
Abstract
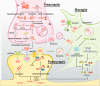
Alzheimer's disease (AD) is the most common neurodegenerative disorder seen in age-dependent dementia. There is currently no effective treatment for AD, which may be attributed in part to lack of a clear underlying mechanism. Studies within the last few decades provide growing evidence for a central role of amyloid β (Aβ) and tau, as well as glial contributions to various molecular and cellular pathways in AD pathogenesis. Herein, we review recent progress with respect to Aβ- and tau-associated mechanisms, and discuss glial dysfunction in AD with emphasis on neuronal and glial receptors that mediate Aβ-induced toxicity. We also discuss other critical factors that may affect AD pathogenesis, including genetics, aging, variables related to environment, lifestyle habits, and describe the potential role of apolipoprotein E (APOE), viral and bacterial infection, sleep, and microbiota. Although we have gained much towards understanding various aspects underlying this devastating neurodegenerative disorder, greater commitment towards research in molecular mechanism, diagnostics and treatment will be needed in future AD research.
Keywords: Alzheimer’s disease; Astrocyte; Aβ; Microglia; Tau.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Alzheimer's A. 2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12(4):459–509. - PubMed
-
- C., P., World Alzheimer Report 2018 . The state of the art of dementia research: new frontiers. London: Alzheimer’s Disease International; 2018.
-
- Prince MJ, WA, Guerchet M, Ali G-C, Wu Y-T, Prina M. World Alzheimer report 2015: the global impact of dementia: an analysis of prevalence, Incidence, Cost and Trends. 2015.
-
- Zubenko GS, et al. A collaborative study of the emergence and clinical features of the major depressive syndrome of Alzheimer's disease. Am J Psychiatry. 2003;160(5):857–866. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

