Follicular lymphoma in the modern era: survival, treatment outcomes, and identification of high-risk subgroups
- PMID: 32678074
- PMCID: PMC7366724
- DOI: 10.1038/s41408-020-00340-z
Follicular lymphoma in the modern era: survival, treatment outcomes, and identification of high-risk subgroups
Abstract
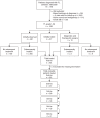
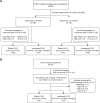
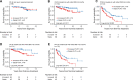
Patients with follicular lymphoma (FL) frequently require multiple treatments during their disease course; however, survival based on lines of treatment remains poorly described in the post-rituximab era. Also, the Follicular Lymphoma International Prognostic Index (FLIPI) score was developed to predict survival at diagnosis, yet it remains unknown whether increase in FLIPI score following an initial observation period is associated with less-favorable outcomes. To address these knowledge gaps, we retrospectively studied 1088 patients with FL grade 1-3A managed between 1998 and 2009 at our institution. Median overall survival (OS) and progression-free survival (PFS) after first-line treatment were not reached and 4.73 years, respectively. Following successive lines of treatment, years of median OS and PFS were, respectively: after second-line, 11.7 and 1.5; third-line, 8.8 and 1.1; fourth-line, 5.3 and 0.9; fifth-line, 3.1 and 0.6; sixth-line, 1.9 and 0.5. In initially observed, subsequently treated patients, FLIPI score increase after observation was associated with inferior survival following first-line treatment. The reduced survival we observed after second-line and later therapy supports the development of new treatments for relapsed patients and benchmarks historical targets for clinical endpoints. This study also highlights the utility of changes in FLIPI score at diagnosis and after observation in identifying patients likely to have worse outcomes.
Conflict of interest statement
C.L.B. has received research support from Janssen, Novartis, Epizyme, Xynomic, and Bayer; has received honoraria from Dava Oncology; and has a consultancy role with Kite Pharma and Juno Therapeutics. J.D.S. has received research support from Genentech/Roche, BeiGene, TG Therapeutics, and BostonGene; and has a consultancy role with AbbVie, AstraZeneca, and Verastem. L.F. has received research support from Roche. P.A.H. has received research support from Portola Pharmaceuticals, Molecular Templates, Incyte, and J&J Pharmaceuticals; and has a consultancy role with Portola Pharmaceuticals, Celgene, Karyopharm Therapeutics, and Juno Therapeutics. S.M.H. has received research support from ADC Therapeutics, Aileron Therapeutics, Celgene, Forty Seven, Infinity Pharmaceuticals/Verastem Oncology, Kyowa Hakko Kirin, Millennium Pharmaceuticals/Takeda Oncology, Seattle Genetics, and Trillium; and has a consultancy role with ADC Therapeutics, Aileron, Corvus Pharmaceuticals, Forty Seven, Innate Pharma, Kyowa Hakko Kirin, Millennium/Takeda, Mundipharma, Portola, and Seattle Genetics. A.K. has received research support from AbbVie, Adaptive Biotechnologies, Celgene, Pharmacyclics, and Seattle Genetics; and has an advisory role with Celgene. M.J.M. has received research support from Genentech, Roche, GlaxoSmithKline, Bayer, Pharmacyclics, Janssen, Rocket Medical, and Seattle Genetics; has received honoraria from Genentech, Roche, Bayer, Pharmacyclics, Janssen, Seattle Genetics, and GlaxoSmithKline; and has a consultancy role with Genentech, Bayer, Merck, Juno, Roche, Teva, Rocket Medical, and Seattle Genetics. A.J.M. has received research support from Incyte, Seattle Genetics, Bristol-Myers Squibb, and Merck; and has a consultancy role with Kyowa Hakko Kirin Pharma, Miragen Therapeutics, Takeda Pharmaceuticals, ADC Therapeutics, Seattle Genetics, Cell Medica, Bristol-Myers Squibb, and Erytech Pharma. C.H.M. has received research support from Bristol-Myers Squibb, Merck, and Seattle Genetics; has a consultancy role with AstraZeneca, Bristol-Myers Squibb, Karyopharm Therapeutics, Merck, Seattle Genetics, Takeda, and Vaniam Group; and has an advisory role with AstraZeneca, Karyopharm Therapeutics, Merck, Seattle Genetics, Takeda, and Vaniam Group. A. Noy has received research support from Pharmacyclics and Rafael Pharmaceuticals; has a consultancy role with Morphoysis; and received honoraria from Janssen, Pharmacyclics, Prime Oncology, Medscape, and Targeted Oncology. L.M.P. has received research support from Genentech, Juno, and Regeneron; and has received honoraria from Novartis, Merck, Celgene, Juno, and Pharmacyclics. D.S. has an advisory role with Seattle Genetics. G.v.K. has received research support from Pharmacyclics, Genentech, and Bayer. A.D.Z. has received research support from MEI Pharma, MorphoSys, Sandoz, Celgene, Roche, and Gilead Sciences; has a consultancy role with Genentech/Roche, Gilead, Celgene, Janssen, Amgen, Novartis, and Adaptive Biotechnologies; and serves on the board of directors (Data Monitoring Committee Chair) for BeiGene. A.Y. has received research support from Janssen, Curis, Merck, Bristol-Myers Squibb, Syndax Pharmaceuticals, and Roche; has received honoraria from Janssen, AbbVie, Merck, Curis, Epizyme, Roche, and Takeda; and has a consultancy role with BioPath, Xynomic, Epizyme, and Roche. The remaining authors (F.S., A.A., A. Ni, K.S., Z.Y., P.C.C., A.H., E.J., C.O., and V.E.S.) declare no competing financial interests.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

