Vagus nerve stimulation boosts the drive to work for rewards
- PMID: 32678082
- PMCID: PMC7366927
- DOI: 10.1038/s41467-020-17344-9
Vagus nerve stimulation boosts the drive to work for rewards
Abstract
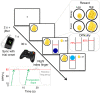
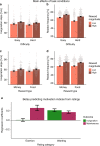
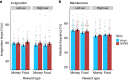
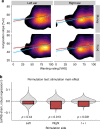
Interoceptive feedback transmitted via the vagus nerve plays a vital role in motivation by tuning actions according to physiological needs. Whereas vagus nerve stimulation (VNS) reinforces actions in animals, motivational effects elicited by VNS in humans are still largely elusive. Here, we applied non-invasive transcutaneous auricular VNS (taVNS) on the left or right ear while participants exerted effort to earn rewards using a randomized cross-over design (vs. sham). In line with preclinical studies, acute taVNS enhances invigoration of effort, and stimulation on the left side primarily facilitates invigoration for food rewards. In contrast, we do not find conclusive evidence that acute taVNS affects effort maintenance or wanting ratings. Collectively, our results suggest that taVNS enhances reward-seeking by boosting invigoration, not effort maintenance and that the stimulation side affects generalization beyond food reward. Thus, taVNS may enhance the pursuit of prospective rewards which may pave avenues to treat motivational deficiencies.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Non-invasive vagus nerve stimulation and the motivation to work for rewards: A replication of Neuser et al. (2020, Nature Communications).Psychophysiology. 2024 Apr;61(4):e14484. doi: 10.1111/psyp.14484. Epub 2023 Nov 9. Psychophysiology. 2024. PMID: 37942809 Clinical Trial.
-
Non-invasive vagus nerve stimulation conditions increased invigoration and wanting in depression.Compr Psychiatry. 2024 Jul;132:152488. doi: 10.1016/j.comppsych.2024.152488. Epub 2024 Apr 16. Compr Psychiatry. 2024. PMID: 38657358 Clinical Trial.
-
Acute vagus nerve stimulation does not affect liking or wanting ratings of food in healthy participants.Appetite. 2022 Feb 1;169:105813. doi: 10.1016/j.appet.2021.105813. Epub 2021 Nov 17. Appetite. 2022. PMID: 34798227
-
Does transcutaneous auricular vagus nerve stimulation affect vagally mediated heart rate variability? A living and interactive Bayesian meta-analysis.Psychophysiology. 2021 Nov;58(11):e13933. doi: 10.1111/psyp.13933. Epub 2021 Sep 2. Psychophysiology. 2021. PMID: 34473846 Review.
-
Transcutaneous Auricular Vagus Nerve Stimulation: From Concept to Application.Neurosci Bull. 2021 Jun;37(6):853-862. doi: 10.1007/s12264-020-00619-y. Epub 2020 Dec 23. Neurosci Bull. 2021. PMID: 33355897 Free PMC article. Review.
Cited by
-
How we decide what to eat: Toward an interdisciplinary model of gut-brain interactions.Wiley Interdiscip Rev Cogn Sci. 2022 Jan;13(1):e1562. doi: 10.1002/wcs.1562. Epub 2021 May 11. Wiley Interdiscip Rev Cogn Sci. 2022. PMID: 33977675 Free PMC article. Review.
-
Transcutaneous Auricular Vagus Stimulation Improves Gait and Reaction Time in Parkinson's Disease.Mov Disord. 2022 Oct;37(10):2163-2164. doi: 10.1002/mds.29166. Epub 2022 Jul 21. Mov Disord. 2022. PMID: 35861362 Free PMC article. No abstract available.
-
tVNS Increases Liking of Orally Sampled Low-Fat Foods: A Pilot Study.Front Hum Neurosci. 2020 Nov 27;14:600995. doi: 10.3389/fnhum.2020.600995. eCollection 2020. Front Hum Neurosci. 2020. PMID: 33328943 Free PMC article.
-
A pooled analysis of the side effects of non-invasive Transcutaneous Auricular Vagus Nerve Stimulation (taVNS).Front Hum Neurosci. 2025 Feb 5;19:1539416. doi: 10.3389/fnhum.2025.1539416. eCollection 2025. Front Hum Neurosci. 2025. PMID: 39981126 Free PMC article.
-
Transcutaneous Vagus Nerve Stimulation Enhances Probabilistic Learning.Psychophysiology. 2025 Mar;62(3):e70037. doi: 10.1111/psyp.70037. Psychophysiology. 2025. PMID: 40059064 Free PMC article.
References
-
- Phillips PE, Walton ME, Jhou TC. Calculating utility: preclinical evidence for cost-benefit analysis by mesolimbic dopamine. Psychopharmacology. 2007;191:483–495. - PubMed
-
- Gigerenzer, G. Gut Feelings: The Intelligence of the Unconscious. (Penguin, Park Imperial, 2007).
-
- Han W, et al. A neural circuit for gut-induced reward. Cell. 2018;175:e623. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

