Inherited cardiac arrhythmias
- PMID: 32678103
- PMCID: PMC7935690
- DOI: 10.1038/s41572-020-0188-7
Inherited cardiac arrhythmias
Abstract
The main inherited cardiac arrhythmias are long QT syndrome, short QT syndrome, catecholaminergic polymorphic ventricular tachycardia and Brugada syndrome. These rare diseases are often the underlying cause of sudden cardiac death in young individuals and result from mutations in several genes encoding ion channels or proteins involved in their regulation. The genetic defects lead to alterations in the ionic currents that determine the morphology and duration of the cardiac action potential, and individuals with these disorders often present with syncope or a life-threatening arrhythmic episode. The diagnosis is based on clinical presentation and history, the characteristics of the electrocardiographic recording at rest and during exercise and genetic analyses. Management relies on pharmacological therapy, mostly β-adrenergic receptor blockers (specifically, propranolol and nadolol) and sodium and transient outward current blockers (such as quinidine), or surgical interventions, including left cardiac sympathetic denervation and implantation of a cardioverter-defibrillator. All these arrhythmias are potentially life-threatening and have substantial negative effects on the quality of life of patients. Future research should focus on the identification of genes associated with the diseases and other risk factors, improved risk stratification and, in particular for Brugada syndrome, effective therapies.
Conflict of interest statement
Competing interests
M.J.A. is a consultant for Audentes Therapeutics, Boston Scientific, Gilead Sciences, Invitae, Medtronic, MyoKardia and St. Jude Medical, and holds equity/royalties of AliveCor, Blue Ox Health and StemoniX. C.A. is a consultant for Novartis Institutes for BioMedical Research, Inc. and Trevena, Inc. All other authors declare no competing interests.
Figures

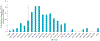



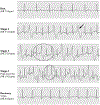
References
-
- Meyer L et al. Incidence, causes, and survival trends from cardiovascular-related sudden cardiac arrest in children and young adults 0 to 35 years of age: a 30-year review. Circulation. 126, 1363–1372 (2012). - PubMed
-
- Winkel BG et al. Nationwide study of sudden cardiac death in persons aged 1–35 years. Eur. Heart J 32, 983–990 (2011). - PubMed
-
- Bardai A et al. Incidence, causes, and outcomes of out-of-hospital cardiac arrest in children. A comprehensive, prospective, population-based study in the Netherlands. J. Am. Coll. Cardiol 57, 1822–1828 (2011). - PubMed
-
- Bagnall RD et al. A prospective study of sudden cardiac death among children and young adults. N. Engl. J. Med 374, 2441–2452 (2016). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

