Assembly formation of minor dihydrosphingomyelin in sphingomyelin-rich ordered membrane domains
- PMID: 32678223
- PMCID: PMC7366691
- DOI: 10.1038/s41598-020-68688-7
Assembly formation of minor dihydrosphingomyelin in sphingomyelin-rich ordered membrane domains
Abstract
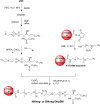
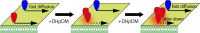
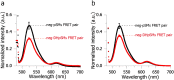
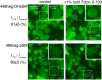
The lipidome of mammalian cells not only contain sphingomyelin (SM) but also, as a minor component, dihydrosphongomyelin (DHSM), in which the double bond at C4-C5 in the sphingosine base is reduced to a single-bond linkage. It has been indicated that DHSM forms ordered domains more effectively than SM due to its greater potential to induce intermolecular hydrogen bonds. However, direct information on partition and dynamic behaviors of DHSM in raft-like liquid-ordered (Lo) and non-raft-like liquid-disordered (Ld) phase-segregated membranes has been lacking. In the present study, we prepared fluorescent derivatives of DHSM and compared their behaviors to those of fluorescent SM and phosphatidylcholine (PC) derivatives. Fluorescence microscopy showed that DHSM is more preferentially localized to the Lo domains in the Lo/Ld phase-segregated giant unilamellar vesicles than SM and PC. Most importantly, diffusion coefficient measurements indicated that DHSM molecules form DHSM-condensed assembly inside the SM-rich Lo domain of the SM/dioleoylphosphatidylcholine/cholesterol system even when DHSM accounts for 1-3.3 mol% of total lipids. Such heterogeneous distribution of DHSM in the SM-rich Lo domains was further confirmed by inter-lipid FRET experiments. This study provides new insights into the biological functions and significance of minor component DHSM in lipid rafts.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

