Facile formulation and fabrication of the cathode using a self-lithiated carbon for all-solid-state batteries
- PMID: 32678243
- PMCID: PMC7367347
- DOI: 10.1038/s41598-020-68865-8
Facile formulation and fabrication of the cathode using a self-lithiated carbon for all-solid-state batteries
Abstract
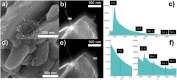
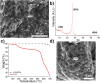
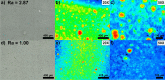
We propose a innovative concept to boost the electrochemical performance of cathode composite electrodes using surface-modified carbons with hydrophilic moieties to increase their dispersion in a Lithium Nickel Manganese Cobalt Oxide (NMC) cathode and in-situ generate Li-rich carbon surfaces. Using a rapid aqueous process, the hydrophilic carbon is effectively dispersed in NMC particles followed by the conversion of its acid surface groups (e.g. -COOH), which interact with the NMC particles due to their basicity, into grafted Li salt (-COO-Li+). The solid-state batteries prepared using the cathode composites with surface-modified carbon exhibit better electrochemical performance. Such modified carbons led to a better electronic conduction path as well as facilitating Li+ ions transfer at the carbon/NMC interface due to the presence of lithiated carboxylate groups on their surface.
Conflict of interest statement
The authors declare no competing interests.
Figures
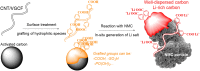





References
-
- Chu S, Cui Y, Liu N. The path towards sustainable energy. Nat. Mater. 2016;16:16–22. - PubMed
-
- Ellis BL, Lee KT, Nazar LF. Positive electrode materials for Li-ion and Li-batteries. Chem. Mater. 2010;22:691–714.
-
- Sun C, Liu J, Gong Y, Wilkinson DP, Zhang J. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy. 2017;33:363–386.
-
- Myung S-T, et al. Nickel-rich layered cathode materials for automotive lithium-ion batteries: Achievements and perspectives. ACS Energy Lett. 2017;2:196–223.
-
- Wood M, et al. Chemical stability and long-term cell performance of low-cobalt, Ni-Rich cathodes prepared by aqueous processing for high-energy Li-Ion batteries. Energy Storage Mater. 2020;24:188–197.
LinkOut - more resources
Full Text Sources

