Nanofiber Technology for Regenerative Engineering
- PMID: 32678581
- PMCID: PMC7484273
- DOI: 10.1021/acsnano.0c03981
Nanofiber Technology for Regenerative Engineering
Abstract
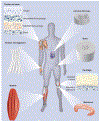
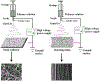
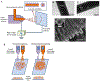
Regenerative engineering is powerfully emerging as a successful strategy for the regeneration of complex tissues and biological organs using a convergent approach that integrates several fields of expertise. This innovative and disruptive approach has spurred the demands for more choice of biomaterials with distinctive biological recognition properties. An ideal biomaterial is one that closely mimics the hierarchical architecture and features of the extracellular matrices (ECM) of native tissues. Nanofabrication technology presents an excellent springboard for the development of nanofiber scaffolds that can have positive interactions in the immediate cellular environment and stimulate specific regenerative cascades at the molecular level to yield healthy tissues. This paper systematically reviews the electrospinning process technology and its utility in matrix-based regenerative engineering, focusing mainly on musculoskeletal tissues. It briefly outlines the electrospinning/three-dimensional printing system duality and concludes with a discussion on the technology outlook and future directions of nanofiber matrices.
Keywords: 3D-printed scaffold; biodegradable polymer; biomaterial−cell interactions; drug delivery; dual-scale matrix; electrospinning; electrospun nanofiber; stem cells; sustained release; tissue regeneration.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
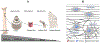

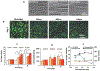


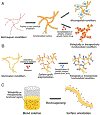
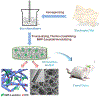
References
-
- Ogueri KS; Laurencin CT Matrix-Based Bone Regenerative Engineering In Encyclopedia of Bone Biology, Zaidi M, Ed. Academic Press: Oxford, 2020; pp 135–148.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

