Burden of Illness Among People with Migraine and ≥ 4 Monthly Headache Days While Using Acute and/or Preventive Prescription Medications for Migraine
- PMID: 32678721
- PMCID: PMC10391061
- DOI: 10.18553/jmcp.2020.20100
Burden of Illness Among People with Migraine and ≥ 4 Monthly Headache Days While Using Acute and/or Preventive Prescription Medications for Migraine
Abstract
Background: Migraine is a chronic disease that reduces health-related quality of life. Little is known about the burden of migraine in individuals who are potential candidates for preventive treatment with ≥ 4 monthly headache days currently using migraine medications.
Objective: To characterize the burden of migraine among patients reporting ≥ 4 monthly headache days while taking acute and/or preventive migraine medications.
Methods: In this retrospective, cross-sectional study, data from the 2016 U.S. National Health and Wellness Survey (N = 97,503) compared the burden of migraine among individuals self-reporting a diagnosis of migraine by a health care professional and ≥ 4 monthly headache days while using acute and/or preventive prescription migraine medications to matched nonmigraine controls. Propensity score matching across different variables (e.g., age, gender, and body mass index) was used to identify matched controls from respondents who did not self-report a diagnosis of migraine. Migraine-associated burden was measured by impairment in work productivity and daily activities (Work Productivity and Activity Impairment questionnaire), all-cause health care resource utilization (HRU), and all-cause direct and indirect costs.
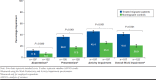
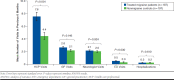
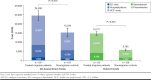
Results: This analysis included 197 treated migraine patients with ≥ 4 monthly headache days and 197 matched nonmigraine controls. Greater proportions of treated migraine patients reported comorbid depression (58.4% vs. 27.9%, P < 0.001) or generalized anxiety disorder (15.2% vs. 8.6%, P = 0.043) and were on long-term disability (13.7% vs. 5.6%, P = 0.003). Absenteeism (11.8% vs. 6.3%, P = 0.030); presenteeism (36.0% vs. 17.5%, P < 0.001); overall work impairment (41.0% vs. 20.9%, P < 0.001); and activity impairment (45.4% vs. 25.4%, P < 0.001) were greater in treated migraine patients versus nonmigraine controls. Treated migraine patients had higher all-cause HRU and higher all-cause direct ($24,499.90 vs. $15,318.91, P = 0.013) and indirect ($14,770.57 vs. $5,764.93, P < 0.001) costs than nonmigraine controls.
Conclusions: Treated migraine patients with ≥ 4 monthly headache days reported significantly reduced work productivity and increased all-cause HRU and cost despite migraine treatment compared with nonmigraine controls. These findings highlight unmet needs in the treatment and management of migraine.
Disclosures: This study was funded by Teva Pharmaceutical Industries (Petach Tikva, Israel). Cohen is an employee of Teva Branded Pharmaceutical Products R&D (USA); Bell was an employee of Teva Pharmaceutical Industries at the time of this study and holds stock/stock options in Teva Pharmaceutical Industries. Lee is an employee of Kantar, which received funding from Teva Pharmaceutical Industries for data analyses performed for this study. Buse has served as a paid consultant to Amgen/Novartis, Allergan, Biohaven, Eli Lilly, Promius/Dr. Reddy's, and Teva Pharmaceuticals, but she was not compensated financially for work on this study. Yugrakh has received research support from Teva Pharmaceuticals and Cefaly Technology. Lipton has received research support from the NIH, the Migraine Research Foundation, and the National Headache Foundation; holds stock options in eNeura Therapeutics and Biohaven Holdings; serves as consultant, advisory board member, or has received honoraria from the American Academy of Neurology, Alder, Allergan, the American Headache Society, Amgen, Autonomic Technologies, Avanir, Biohaven, BioVision, Boston Scientific, Dr. Reddy's, electroCore, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, Pernix, Pfizer, Supernus, Teva, Trigemina, Vector, and Vedanta. This study was presented as a poster at the American Academy of Neurology 2018 Annual Meeting, April 21-27, 2018, in Los Angeles, CA; PAINWeek 2018, September 4-8, 2018, in Las Vegas, NV; and the 2017 European Headache Federation (EHF) Congress, December 1-3, 2017, in Rome, Italy.
Conflict of interest statement
This study was funded by Teva Pharmaceutical Industries (Petach Tikva, Israel). Cohen is an employee of Teva Branded Pharmaceutical Products R&D (USA); Bell was an employee of Teva Pharmaceutical Industries at the time of this study and holds stock/stock options in Teva Pharmaceutical Industries. Lee is an employee of Kantar, which received funding from Teva Pharmaceutical Industries for data analyses performed for this study. Buse has served as a paid consultant to Amgen/Novartis, Allergan, Biohaven, Eli Lilly, Promius/Dr. Reddy’s, and Teva Pharmaceuticals, but she was not compensated financially for work on this study. Yugrakh has received research support from Teva Pharmaceuticals and Cefaly Technology. Lipton has received research support from the NIH, the Migraine Research Foundation, and the National Headache Foundation; holds stock options in eNeura Therapeutics and Biohaven Holdings; serves as consultant, advisory board member, or has received honoraria from the American Academy of Neurology, Alder, Allergan, the American Headache Society, Amgen, Autonomic Technologies, Avanir, Biohaven, BioVision, Boston Scientific, Dr. Reddy’s, electroCore, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, Pernix, Pfizer, Supernus, Teva, Trigemina, Vector, and Vedanta.
This study was presented as a poster at the American Academy of Neurology 2018 Annual Meeting, April 21-27, 2018, in Los Angeles, CA; PAINWeek 2018, September 4-8, 2018, in Las Vegas, NV; and the 2017 European Headache Federation (EHF) Congress, December 1-3, 2017, in Rome, Italy.
Figures
References
-
- Institute for Health Metrics and Evaluation. Global Burden of Diseases, Injuries, and Risk Factors Study (GBD). 2018. Available at: http://vizhub.healthdata.org/gbd-compare. Accessed June 16, 2020.
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789-858. - PMC - PubMed
-
- Bonafede M, Cai Q, Cappell K, et al. . Factors associated with direct health care costs among patients with migraine. J Manag Care Spec Pharm. 2017;23(11):1169-76. Available at: https://www.jmcp.org/doi/10.18553/jmcp.2017.23.11.1169. - DOI - PMC - PubMed
-
- Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41(7):646-57. - PubMed
-
- Munakata J, Hazard E, Serrano D, et al. . Economic burden of transformed migraine: results from the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2009;49(4):498-508. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous