Orchestrating Opiate-Associated Memories in Thalamic Circuits
- PMID: 32679036
- PMCID: PMC8130576
- DOI: 10.1016/j.neuron.2020.06.028
Orchestrating Opiate-Associated Memories in Thalamic Circuits
Erratum in
-
Orchestrating Opiate-Associated Memories in Thalamic Circuits.Neuron. 2022 Oct 19;110(20):3406. doi: 10.1016/j.neuron.2022.09.031. Neuron. 2022. PMID: 36265443 Free PMC article. No abstract available.
Abstract
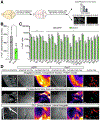
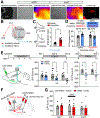
Disrupting memories that associate environmental cues with drug experiences holds promise for treating addiction, yet accessing the distributed neural network that stores such memories is challenging. Here, we show that the paraventricular nucleus of the thalamus (PVT) orchestrates the acquisition and maintenance of opiate-associated memories via projections to the central nucleus of the amygdala (CeA) and nucleus accumbens (NAc). PVT→CeA activity associates morphine reward to the environment, whereas transient inhibition of the PVT→NAc pathway during retrieval causes enduring protection against opiate-primed relapse. Using brain-wide activity mapping, we revealed distributed network activities that are altered in non-relapsing mice, which enabled us to find that activating the downstream NAc→lateral hypothalamus (LH) pathway also prevents relapse. These findings establish the PVT as a key node in the opiate-associated memory network and demonstrate the potential of targeting the PVT→NAc→LH pathway for treating opioid addiction.
Keywords: central nucleus of amygdala; memory; nucleus accumbens; opiate; paraventricular nucleus of the thalamus; reconsolidation; relapse; withdrawal.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures



Comment in
-
Thalamic Retrieval of Opioid Memories.Neuron. 2020 Sep 23;107(6):992-994. doi: 10.1016/j.neuron.2020.09.006. Neuron. 2020. PMID: 32971000 Free PMC article.
References
-
- Boyden ES, Zhang F, Bamberg E, Nagel G, and Deisseroth K (2005). Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 8, 1263–1268. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

