Targeting Two-Pore Channels: Current Progress and Future Challenges
- PMID: 32679067
- PMCID: PMC7365084
- DOI: 10.1016/j.tips.2020.06.002
Targeting Two-Pore Channels: Current Progress and Future Challenges
Abstract
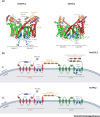
Two-pore channels (TPCs) are cation-permeable channels located on endolysosomal membranes and important mediators of intracellular Ca2+ signalling. TPCs are involved in various pathophysiological processes, including cell growth and development, metabolism, and cancer progression. Most studies of TPCs have used TPC-/- cell or whole-animal models, or Ned-19, an indirect inhibitor. The TPC activation mechanism remains controversial, which has made it difficult to develop selective modulators. Recent studies of TPC structure and their interactomes are aiding the development of direct pharmacological modulators. This process is still in its infancy, but will facilitate future research and TPC targeting for therapeutical purposes. Here, we review the progress of current research into TPCs, including recent insights into their structures, functional roles, mechanisms of activation, and pharmacological modulators.
Keywords: NAADP; calcium; cell signalling; endolysosomes; interactome; two-pore channels.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures

References
-
- Parrington J., Tunn R. Ca 2+ signals, NAADP and two-pore channels: role in cellular differentiation. Acta Physiol. 2014;211:285–296. - PubMed
-
- Berridge M.J., et al. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003;4:517–529. - PubMed
-
- Lee H.C., Aarhus R. A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J. Biol. Chem. 1995;270:2152–2157. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

