Molecular epidemiology of resistance to antimalarial drugs in the Greater Mekong subregion: an observational study
- PMID: 32679084
- PMCID: PMC7689289
- DOI: 10.1016/S1473-3099(20)30228-0
Molecular epidemiology of resistance to antimalarial drugs in the Greater Mekong subregion: an observational study
Abstract
Background: The Greater Mekong subregion is a recurrent source of antimalarial drug resistance in Plasmodium falciparum malaria. This study aimed to characterise the extent and spread of resistance across this entire region between 2007 and 2018.
Methods: P falciparum isolates from Myanmar, Thailand, Laos, and Cambodia were obtained from clinical trials and epidemiological studies done between Jan 1, 2007, and Dec 31, 2018, and were genotyped for molecular markers (pfkelch, pfcrt, pfplasmepsin2, and pfmdr1) of antimalarial drug resistance. Genetic relatedness was assessed using microsatellite and single nucleotide polymorphism typing of flanking sequences around target genes.
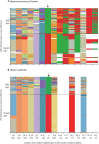
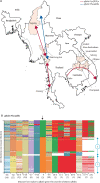
Findings: 10 632 isolates were genotyped. A single long pfkelch Cys580Tyr haplotype (from -50 kb to +31·5 kb) conferring artemisinin resistance (PfPailin) now dominates across the eastern Greater Mekong subregion. Piperaquine resistance associated with pfplasmepsin2 gene amplification and mutations in pfcrt downstream of the Lys76Thr chloroquine resistance locus has also developed. On the Thailand-Myanmar border a different pfkelch Cys580Tyr lineage rose to high frequencies before it was eliminated. Elsewhere in Myanmar the Cys580Tyr allele remains widespread at low allele frequencies. Meanwhile a single artemisinin-resistant pfkelch Phe446Ile haplotype has spread across Myanmar. Despite intense use of dihydroartemisinin-piperaquine in Kayin state, eastern Myanmar, both in treatment and mass drug administrations, no selection of piperaquine resistance markers was observed. pfmdr1 amplification, a marker of resistance to mefloquine, remains at low prevalence across the entire region.
Interpretation: Artemisinin resistance in P falciparum is now prevalent across the Greater Mekong subregion. In the eastern Greater Mekong subregion a multidrug resistant P falciparum lineage (PfPailin) dominates. In Myanmar a long pfkelch Phe446Ile haplotype has spread widely but, by contrast with the eastern Greater Mekong subregion, there is no indication of artemisinin combination therapy (ACT) partner drug resistance from genotyping known markers, and no evidence of spread of ACT resistant P falciparum from the east to the west. There is still a window of opportunity to prevent global spread of ACT resistance.
Funding: Thailand Science Research and Innovation, Initiative 5%, Expertise France, Wellcome Trust.
Copyright © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures




Comment in
-
Knowing the enemy: genetics to track antimalarial resistance.Lancet Infect Dis. 2020 Dec;20(12):1361-1362. doi: 10.1016/S1473-3099(20)30271-1. Epub 2020 Jul 14. Lancet Infect Dis. 2020. PMID: 32679082 No abstract available.
References
-
- WHO . 3rd edn. World Health Organization; Geneva: 2015. Guidelines for the treatment of malaria.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

