Enhanced S-Cone Syndrome: Spectrum of Clinical, Imaging, Electrophysiologic, and Genetic Findings in a Retrospective Case Series of 56 Patients
- PMID: 32679203
- PMCID: PMC7861019
- DOI: 10.1016/j.oret.2020.07.008
Enhanced S-Cone Syndrome: Spectrum of Clinical, Imaging, Electrophysiologic, and Genetic Findings in a Retrospective Case Series of 56 Patients
Abstract
Purpose: To describe the detailed phenotype, long-term clinical course, clinical variability, and genotype of patients with enhanced S-cone syndrome (ESCS).
Design: Retrospective case series.
Participants: Fifty-six patients with ESCS.
Methods: Clinical history, examination, imaging, and electrophysiologic findings of 56 patients (age range, 1-75 years) diagnosed with ESCS were reviewed. Diagnosis was established by molecular confirmation of disease-causing variants in the NR2E3 gene (n = 38) or by diagnostic full-field electroretinography findings (n = 18).
Main outcome measures: Age at onset of visual symptoms, best-corrected visual acuity (BCVA), quantitative age-related electrophysiologic decline, and imaging findings.
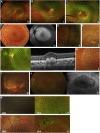
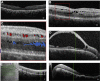
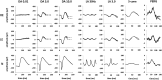
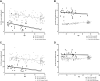
Results: Mean age at onset of visual symptoms was 4.0 years, and median age at presentation was 20.5 years, with mean follow-up interval being 6.1 years. Six patients were assessed once. Disease-causing variants in NR2E3 were identified in 38 patients. Mean BCVA of the better-seeing eye was 0.32 logarithm of the minimum angle of resolution (logMAR) at baseline and 0.39 logMAR at follow-up. In most eyes (76% [76/100]), BCVA remained stable, with a mean BCVA change of 0.07 logMAR during follow-up. Nyctalopia was the most common initial symptom, reported in 92.9% of patients (52/56). Clinical findings were highly variable and included foveomacular schisis (41.1% [26/56]), yellow-white dots (57.1% [32/56]), nummular pigmentation (85.7% [48/56]), torpedo-like lesions (10.7% [6/56]), and circumferential subretinal fibrosis (7.1% [4/56]). Macular and peripheral patterns of autofluorescence were classified as (1) minimal change, (2) hypoautofluorescent (mild diffuse, moderate speckled, moderate diffuse, or advanced), or (3) hyperautofluorescent flecks. One patient showed undetectable electroretinography findings; quantification of main electroretinography components in all other patients revealed amplitude and peak time variability but with pathognomonic electroretinography features. The main electroretinography components showed evidence of age-related worsening over 6.7 decades, at a rate indistinguishable from that seen in unaffected control participants. Eighteen sequence variants in NR2E3 were identified, including 4 novel missense changes.
Conclusions: Enhanced S-cone syndrome has a highly variable phenotype with relative clinical and imaging stability over time. Most electroretinography findings have pathognomonic features, but quantitative assessment reveals variability and a normal mean rate of age-related decline, consistent with largely nonprogressive peripheral retinal dysfunction.
Keywords: Electroretinography; Enhanced S-cone syndrome; Molecular genetics; NR2E3.
Crown Copyright © 2020. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Schorderet D.F., Escher P. NR2E3 mutations in enhanced S-cone sensitivity syndrome (ESCS), Goldmann-Favre syndrome (GFS), clumped pigmentary retinal degeneration (CPRD), and retinitis pigmentosa (RP) Hum Mutat. 2009;30:1475–1485. - PubMed
-
- Gerber S., Rozet J.M., Takezawa S.I. The photoreceptor cell-specific nuclear receptor gene (PNR) accounts for retinitis pigmentosa in the Crypto-Jews from Portugal (Marranos), survivors from the Spanish Inquisition. Hum Genet. 2000;107:276–284. - PubMed
-
- Sharon D., Sandberg M.A., Berson E.L., Dryja T.P. Shared mutations in NR2E3 in enhanced S-cone syndrome, Goldmann-Favre syndrome, and clumped pigmentary retinal degeneration. Invest Ophthalmol Vis Sci. 2002;43 1316–1323. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

